Packages (Simulation)

Reagent Preparation
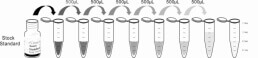
Image (I)
Image (II)
Certificate


Mini Samples ELISA Kit for Aspartate Aminotransferase (AST)
cCAT; AAT; ASAT; SGOT; GOT1; Cysteine transaminase, cytoplasmic; Transaminase A; Aspartate Transaminase 1,Cytoplasmic; Glutamic-Oxaloacetic Transaminase 1,Soluble
- Product No.MEB214Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- Sample Typeserum, plasma, tissue homogenates, cell lysates, cell culture supernates and other biological fluids
- Test MethodDouble-antibody Sandwich
- Assay Length3h
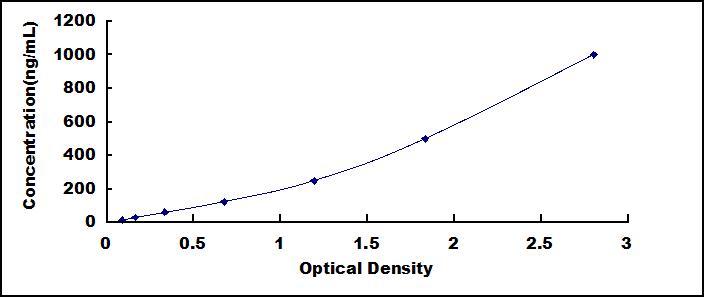
- Detection Range15.6-1,000ng/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 5.5ng/mL.
- DownloadInstruction Manual
- UOM 48T96T 96T*5 96T*10 96T*100
- FOB
US$ 559
US$ 798
US$ 3591
US$ 6783
US$ 55860
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Mini Samples Aspartate Aminotransferase (AST).
No significant cross-reactivity or interference between Mini Samples Aspartate Aminotransferase (AST) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Mini Samples Aspartate Aminotransferase (AST) and the recovery rates were calculated by comparing the measured value to the expected amount of Mini Samples Aspartate Aminotransferase (AST) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 80-88 | 80 |
| EDTA plasma(n=5) | 84-98 | 94 |
| heparin plasma(n=5) | 85-97 | 89 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Mini Samples Aspartate Aminotransferase (AST) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Mini Samples Aspartate Aminotransferase (AST) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Mini Samples Aspartate Aminotransferase (AST) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 97-105% | 80-96% | 80-90% | 96-103% |
| EDTA plasma(n=5) | 84-97% | 98-105% | 95-103% | 86-101% |
| heparin plasma(n=5) | 87-101% | 95-103% | 87-101% | 98-105% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
| Standard | 2 | Standard Diluent | 1×20mL |
| Detection Reagent A | 1×60µL | Assay Diluent A | 1×6mL |
| Detection Reagent B | 1×60µL | Assay Diluent B | 1×6mL |
| TMB Substrate | 1×4.5mL | Stop Solution | 1×3mL |
| Wash Buffer (30 × concentrate) | 1×10mL | Instruction manual | 1 |
Assay procedure summary
1. Prepare all reagents, samples and standards;
2. Add 25µL standard or sample to each well. Incubate 1 hour at 37°C;
3. Aspirate and add 25µL prepared Detection Reagent A. Incubate 1 hour at 37°C;
4. Aspirate and wash 3 times;
5. Add 25µL prepared Detection Reagent B. Incubate 30 minutes at 37°C;
6. Aspirate and wash 5 times;
7. Add 25µL Substrate Solution. Incubate 10-20 minutes at 37°C;
8. Add 20µL Stop Solution. Read at 450nm immediately.
GIVEAWAYS
INCREMENT SERVICES
| Magazine | Citations |
| Kidney International | Adrenocorticotropic hormone ameliorates acute kidney injury by steroidogenic-dependent and -independent mechanisms PubMed: PMC3612362 |
| Proceedings of the National Academy of Sciences | Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model PubMed: PMC3382536 |
| African Journal of Pharmacy and Pharmacology | Protective effect of Panax ginseng against N-acetyl-p-aminophenol-induced hepatotoxicity in rats Academicjournals: Source |
| Kidney International | Adrenocorticotropic hormone ameliorates acute kidney injury by steroidogenic-dependent and-independent mechanisms Pubmed: 23325074 |
| European Journal of Pharmacology | Protective effect of Et-1 receptor antagonist bosentan on paracetamol induced acute liver toxicity in rats ScienceDirect: S0014299914000235 |
| Journal of Immunology | The P2X1 Receptor Is Required for Neutrophil Extravasation during Lipopolysaccharide-Induced Lethal Endotoxemia in Mice Pubmed:25480563 |
| Gastroenterol Res Pract | Assessing the Effect of Leptin on Liver Damage in Case of Hepatic Injury Associated with Paracetamol Poisoning PubMed: 26697061 |
| Gastroenterol Res Pract. | Advanced Studies in Clinical and Experimental Research in Gastroenterology Pubmed:26955389 |
| Scientific Reports | Adiponectin protects the rats liver against chronic intermittent hypoxia induced injury throughAMP-activated protein kinase pathway. pubmed:27678302 |
| Biochimica et Biophysica Acta (BBA)-General Subjects | Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats pubmed:28341485 |
| Scientific Reports | Deletion of MCP-1 Impedes Pathogenesis of Acid Ceramidase Deficiency Pubmed:29379059 |
| FEBS Open Bio | A critical role of hepatitis B virus polymerase in cirrhosis, hepatocellular carcinoma, and steatosis Pubmed:29321963 |
| BMC Complementary and Alternative Medicine | Hepatoprotective activity of Erythrina× neillii leaf extract and characterization of its phytoconstituents Pubmed: 28095910 |
| laboratory investigation | Hepatic pathology and altered gene transcription in a murine model of acid ceramidase deficiency Pubmed: 31186526 |
| Brain Behavior and Immunity | Corticosterone-mediated microglia activation affects dendritic spine plasticity and motor learning functions in minimal hepatic encephalopathy Pubmed: 31437533 |
| Biomed Research International | Liraglutide Attenuates Nonalcoholic Fatty Liver Disease by Modulating Gut Microbiota in Rats Administered a High-Fat Diet Pubmed: 32149099 |
| Nanomaterials | Biological Safety and Biodistribution of Chitosan Nanoparticles Pubmed: 32340313 |
| European Journal of Biology | Effects of Tempol in Lipopolysaccharide-Induced Liver Injury |
| Pathogens | Antimalarial Effect of the Total Glycosides of the Medicinal Plant, Ranunculus japonicus 33925018 |
| Int Immunopharmacol | Taraxasterol mitigates Con A-induced hepatitis in mice by suppressing interleukin-2 expression and its signaling in T lymphocytes 34848154 |
| Acta Pharmacol Sin | NFAT inhibitor 11R-VIVIT ameliorates mouse renal fibrosis after ischemia-reperfusion-induced acute kidney injury 34937917 |
| J Magn Reson Imaging | Water Specific MRI T1 Mapping for Evaluating Liver Inflammation Activity Grades in Rats With Methionine‐Choline‐Deficient Diet‐Induced Nonalcoholic Fatty Liver … Pubmed:35212074 |
| Medcomm | Blockade of mIL‐6R alleviated lipopolysaccharide‐induced systemic inflammatory response syndrome by suppressing NF‐κB‐mediated Ccl2 expression and … Pubmed:35548710 |
| International Immunopharmacology | Dimethyl fumarate ameliorates autoimmune hepatitis in mice by blocking NLRP3 inflammasome activation Pubmed:35605433 |