Packages (Simulation)

Reagent Preparation
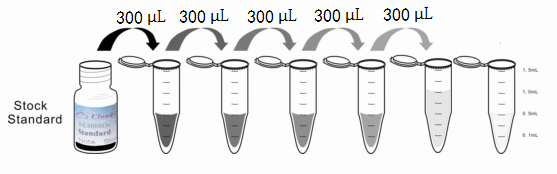
Image (I)
Image (II)
Certificate


Multiplex Assay Kit for Interleukin 17F (IL17F) ,etc. by FLIA (Flow Luminescence Immunoassay)
ML-1
(Note: Up to 8-plex in one testing reaction)
- Product No.LMB955Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- Sample TypeSerum, plasma, tissue homogenates, cell lysates, cell culture supernates and other biological fluids
- Test MethodDouble-antibody Sandwich
- Assay Length3.5h
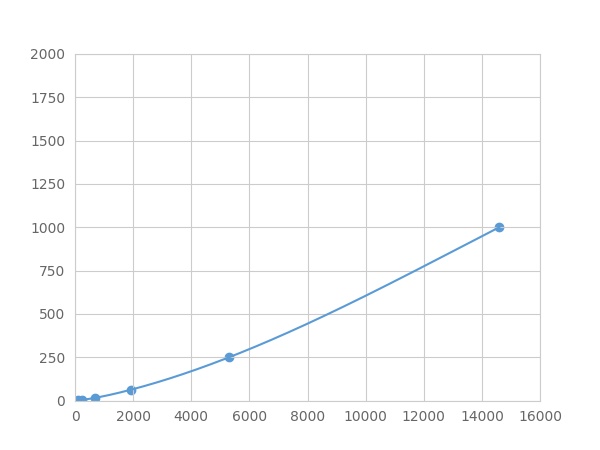
- Detection Range0.98-1000pg/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 0.327 pg/mL.
- DownloadInstruction Manual
- UOM 8Plex 7Plex 6Plex 5Plex 4Plex 3Plex 2Plex1Plex
- FOB
US$ 415
US$ 431
US$ 455
US$ 487
US$ 519
US$ 567
US$ 638
US$ 798
Add to Price Calculator
Result
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Interleukin 17F (IL17F) ,etc. by FLIA (Flow Luminescence Immunoassay).
No significant cross-reactivity or interference between Interleukin 17F (IL17F) ,etc. by FLIA (Flow Luminescence Immunoassay) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Interleukin 17F (IL17F) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Interleukin 17F (IL17F) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 98-105 | 102 |
| EDTA plasma(n=5) | 98-105 | 101 |
| heparin plasma(n=5) | 92-105 | 101 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Interleukin 17F (IL17F) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Interleukin 17F (IL17F) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Interleukin 17F (IL17F) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 82-104% | 90-98% | 82-98% | 86-96% |
| EDTA plasma(n=5) | 83-104% | 80-89% | 78-90% | 94-101% |
| heparin plasma(n=5) | 80-101% | 85-95% | 80-104% | 78-99% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:IL17F) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
GIVEAWAYS
INCREMENT SERVICES
| Magazine | Citations |
| Cytokine | Expression of IL-17A and IL-17F in lipopolysaccharide-induced acute lung injury and the counteraction of anisodamine or methylprednisolone ScienceDirect: S1043466614000040 |
| Medical Sciences | Cerebrospinal Fluid (CSF) IL-17A, IL-17F, IL-34 and CXCL-13 Levels in Amyotrophic Lateral Sclerosis (ALS/MND) Patients |
| Revista da Associacao Medica Brasileira | A comparison of IL-17 and IL-34 concentrations in the cerebrospinal fluid of patients with acute inflammatory demyelinating neuropathy and chronic … |
| Hum Exp Toxicol | Chrysene accelerates the proceeding of chronic obstructive pulmonary disease with the aggravation of inflammation and apoptosis in cigarette smoke exposed mice 33345606 |
| Rev Assoc Med Bras | A comparison of IL-17 and IL-34 concentrations in the cerebrospinal fluid of patients with acute inflammatory demyelinating neuropathy and chronic?¡ 33295414 |
| J Periodontol | Cytokine profile in serum and gingival crevicular fluid of children with inflammatory bowel disease: A case‐control study 34730850 |
| Catalog No. | Related products for research use of Homo sapiens (Human) Organism species | Applications (RESEARCH USE ONLY!) |
| APB955Hu61 | Active Interleukin 17F (IL17F) | Cell culture; Activity Assays. |
| EPB955Hu61 | Eukaryotic Interleukin 17F (IL17F) | Positive Control; Immunogen; SDS-PAGE; WB. |
| RPB955Hu01 | Recombinant Interleukin 17F (IL17F) | Positive Control; Immunogen; SDS-PAGE; WB. |
| APB955Hu01 | Active Interleukin 17F (IL17F) | Cell culture; Activity Assays. |
| PAB955Hu01 | Polyclonal Antibody to Interleukin 17F (IL17F) | IHC |
| LAB955Hu81 | FITC-Linked Polyclonal Antibody to Interleukin 17F (IL17F) | WB; IHC; ICC; IF. |
| LAB955Hu71 | Biotin-Linked Polyclonal Antibody to Interleukin 17F (IL17F) | WB; IHC; ICC. |
| MAB955Hu22 | Monoclonal Antibody to Interleukin 17F (IL17F) | WB; IHC |
| MAB955Hu23 | Monoclonal Antibody to Interleukin 17F (IL17F) | ICC/IF |
| MAB955Hu21 | Monoclonal Antibody to Interleukin 17F (IL17F) | WB |
| SEB955Hu | ELISA Kit for Interleukin 17F (IL17F) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| SCB955Hu | CLIA Kit for Interleukin 17F (IL17F) | Chemiluminescent immunoassay for Antigen Detection. |
| LMB955Hu | Multiplex Assay Kit for Interleukin 17F (IL17F) ,etc. by FLIA (Flow Luminescence Immunoassay) | FLIA Kit for Antigen Detection. |
| KSB955Hu01 | ELISA Kit DIY Materials for Interleukin 17F (IL17F) | Main materials for "Do It(ELISA Kit) Yourself" |