Mouse Model for Actue Spinal Cord Injury (ASCI)
Traumatic Spinal Cord Injury
- Product No.DSI545Mu01
- Organism SpeciesMus musculus (Mouse) Same name, Different species.
- Prototype SpeciesHuman
- SourceInduced by Surgery
- Model Animal StrainsBalb/c Mice(SPF), healthy, male, age: 8~12weeks
- Modeling GroupingRandomly divided into six group: Control group, Model group, Positive drug group and Test drug group.
- Modeling Period4-6 weeks
- Modeling MethodICR mice were anesthetized and laminectomy was performed to expose the spinal cord at t9-T10. Then a spinal cord shock device was used to exert 90 kilodynes of force at the exposed spinal cord to cause contusion-type spinal cord injury. After SCI, gentamicin (8mg/kg) was intramuscularly injected daily for 10 consecutive days to prevent postoperative infection, and urine was pressed with an artificial bladder twice daily until spontaneous bladder activity was resumed (to prevent urinary retention).
One week after treatment, BBB score was performed to evaluate the hind limb locomotion ability of mice.
When BBB score=0, the model is successfully constructed. The mice were divided into two groups with 8 mice in each group. After SCI contusion treatment for 1week, they were injected into the tail vein of rats (only normal saline was injected into the model group, and normal saline containing the tested drugs was injected into the treatment group).
After SCI, 16 mice were fed for 3 weeks. According to the results of 3 weeks after SCI, 3 mice with the most significant effect in each group were selected for spinal cord pathological examination. The remaining 10 mice were continued to be fed and tested for BBB score and mechanical touch induced pain at 5, 7 and 9 weeks. At 9 weeks after SCI, all spinal cord centers were cryopreserved or OCT embedded. - ApplicationsDisease Model
- Downloadn/a
- UOM Each case
- FOB
US$ 260
For more details, please contact local distributors!
Model Evaluation
1. BBB test
BBB tests were performed every other week after injection until 8 weeks after transplantation (i.e., 3week, 5week, 7week, 9week after SCI). Behavioral test data were collected from 16 mice 2 weeks after drug injection (i.e., 3 weeks after SCI), and spinal cord tissues of 3 mice with significant changes (3 mice in each group, i.e., 6 mice) were selected for pathological examination. The remaining 5 mice (5 mice in each group, i.e., 10 mice) underwent BBB test every other week (i.e., 5week, 7week and 9week after SCI). The score ranges from 0 to 21, in which 0 indicates that the hind limb has completely lost its motor function, and 21 indicates that the hind limb has basically normal motor function.
2. Mechanical Allodynia Test
Using the Von Frey pain kit, the pressure of foot contraction was recorded (g) and measured 4 times over a 2-minute interval. The median value obtained after removing the maximum and minimum values was used as the pain threshold (G).
Establishment of spinal cord injury model and postoperative situation of mice:
All mice showed spasmodic tail wagging, retraction and flapping of both lower limbs and body, followed by paralysis of both lower limbs, injury of intact spinal dural, bleeding and edema. After anesthesia, all the mice woke up with paralysis of lower limbs and loss of motor and sensory functions. They moved their bodies with their upper limbs and moved their head and neck normally. 1d after surgery, BBB score of all mice was less than 1 point, activity was significantly reduced, and the amount of food and water was reduced, and most of the mice recovered the spontaneous urination function 7 days after urination.
Mechanical touch induced pain detection: The mice were placed on a metal mesh shelf to adapt to the environment, and the Von Frey wire was used to vertically stimulate the skin surface of the rear paw. The minimum force required to induce positive claw contraction reaction was recorded as the claw contraction threshold, and the PWTs value of the mice after modeling was lower than that of the normal mice.
Pathological Results
3. Immunofluorescence
Two weeks after injection, the expression distribution of MAP-1 was detected by paraffin section of the damaged spinal cord tissue.
4. Microglia/macrophage detection -- laser confocal
ED1 (microglia/macrophage activation marker) was detected in the damaged spinal cord tissue after paraffin section two weeks after drug injection.
Cytokines Level
Statistical Analysis
SPSS software is used for statistical analysis, measurement data to mean ± standard deviation (x ±s), using t test and single factor analysis of variance for group comparison, P<0.05 indicates there was a significant difference, P<0.01 indicates there are very significant differences.
GIVEAWAYS
INCREMENT SERVICES
-
 Tissue/Sections Customized Service
Tissue/Sections Customized Service
-
 Serums Customized Service
Serums Customized Service
-
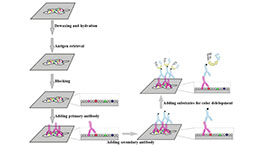 Immunohistochemistry (IHC) Experiment Service
Immunohistochemistry (IHC) Experiment Service
-
 Small Animal In Vivo Imaging Experiment Service
Small Animal In Vivo Imaging Experiment Service
-
 Small Animal Micro CT Imaging Experiment Service
Small Animal Micro CT Imaging Experiment Service
-
 Small Animal MRI Imaging Experiment Service
Small Animal MRI Imaging Experiment Service
-
 Small Animal Ultrasound Imaging Experiment Service
Small Animal Ultrasound Imaging Experiment Service
-
 Transmission Electron Microscopy (TEM) Experiment Service
Transmission Electron Microscopy (TEM) Experiment Service
-
 Scanning Electron Microscope (SEM) Experiment Service
Scanning Electron Microscope (SEM) Experiment Service
-
 Learning and Memory Behavioral Experiment Service
Learning and Memory Behavioral Experiment Service
-
 Anxiety and Depression Behavioral Experiment Service
Anxiety and Depression Behavioral Experiment Service
-
 Drug Addiction Behavioral Experiment Service
Drug Addiction Behavioral Experiment Service
-
 Pain Behavioral Experiment Service
Pain Behavioral Experiment Service
-
 Neuropsychiatric Disorder Behavioral Experiment Service
Neuropsychiatric Disorder Behavioral Experiment Service
-
 Fatigue Behavioral Experiment Service
Fatigue Behavioral Experiment Service
-
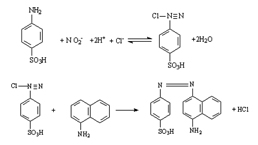 Nitric Oxide Assay Kit (A012)
Nitric Oxide Assay Kit (A012)
-
 Nitric Oxide Assay Kit (A013-2)
Nitric Oxide Assay Kit (A013-2)
-
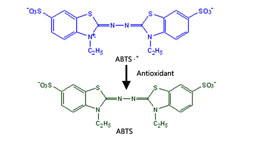 Total Anti-Oxidative Capability Assay Kit(A015-2)
Total Anti-Oxidative Capability Assay Kit(A015-2)
-
 Total Anti-Oxidative Capability Assay Kit (A015-1)
Total Anti-Oxidative Capability Assay Kit (A015-1)
-
 Superoxide Dismutase Assay Kit
Superoxide Dismutase Assay Kit
-
 Fructose Assay Kit (A085)
Fructose Assay Kit (A085)
-
 Citric Acid Assay Kit (A128 )
Citric Acid Assay Kit (A128 )
-
 Catalase Assay Kit
Catalase Assay Kit
-
 Malondialdehyde Assay Kit
Malondialdehyde Assay Kit
-
 Glutathione S-Transferase Assay Kit
Glutathione S-Transferase Assay Kit
-
 Microscale Reduced Glutathione assay kit
Microscale Reduced Glutathione assay kit
-
 Glutathione Reductase Activity Coefficient Assay Kit
Glutathione Reductase Activity Coefficient Assay Kit
-
 Angiotensin Converting Enzyme Kit
Angiotensin Converting Enzyme Kit
-
 Glutathione Peroxidase (GSH-PX) Assay Kit
Glutathione Peroxidase (GSH-PX) Assay Kit
-
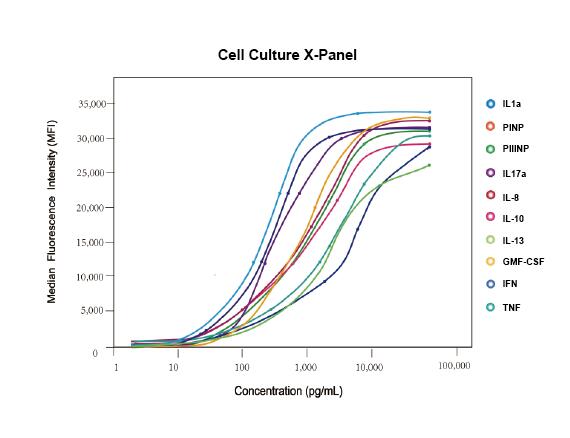 Cloud-Clone Multiplex assay kits
Cloud-Clone Multiplex assay kits
| Catalog No. | Related products for research use of Mus musculus (Mouse) Organism species | Applications (RESEARCH USE ONLY!) |
| DSI545Mu01 | Mouse Model for Actue Spinal Cord Injury (ASCI) | Disease Model |
| TSI545Mu01 | Mouse Heart Tissue of Actue Spinal Cord Injury (ASCI) | Paraffin slides for pathologic research: IHC,IF and HE,Masson and other stainings |
| TSI545Mu03 | Mouse Liver Tissue of Actue Spinal Cord Injury (ASCI) | Paraffin slides for pathologic research: IHC,IF and HE,Masson and other stainings |
| TSI545Mu05 | Mouse Spleen Tissue of Actue Spinal Cord Injury (ASCI) | Paraffin slides for pathologic research: IHC,IF and HE,Masson and other stainings |
| TSI545Mu07 | Mouse Lung Tissue of Actue Spinal Cord Injury (ASCI) | Paraffin slides for pathologic research: IHC,IF and HE,Masson and other stainings |
| TSI545Mu09 | Mouse Kidney Tissue of Actue Spinal Cord Injury (ASCI) | Paraffin slides for pathologic research: IHC,IF and HE,Masson and other stainings |
| TSI545Mu35 | Mouse Spinal Cord Tissue of Actue Spinal Cord Injury (ASCI) | Paraffin slides for pathologic research: IHC,IF and HE,Masson and other stainings |







