Packages (Simulation)

Reagent Preparation
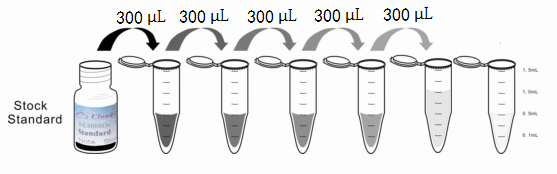
Image (I)
Image (II)
Certificate


Multiplex Assay Kit for Tenascin C (TNC) ,etc. by FLIA (Flow Luminescence Immunoassay)
TN-C; HXB; TN; GMEM; JI; Myotendinous antigen; Neuronectin; GP 150-225; Cytotactin; Hexabrachion; Glioma-associated-extracellular matrix antigen
(Note: Up to 8-plex in one testing reaction)
- Product No.LMB975Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- Sample TypeSerum, plasma, tissue homogenates and other biological fluids.
- Test MethodDouble-antibody Sandwich
- Assay Length3.5h
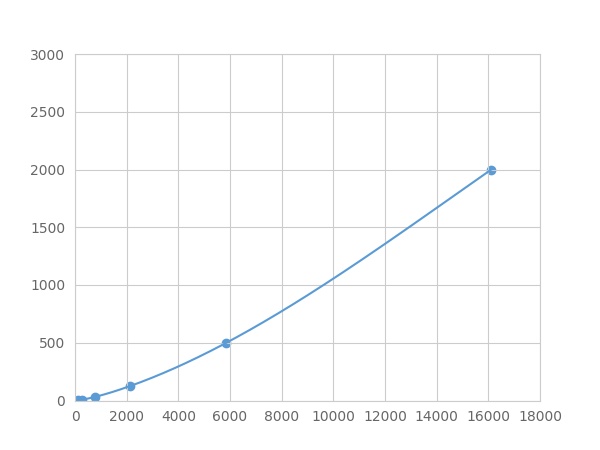
- Detection Range1.95-2000pg/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 0.65 pg/mL.
- DownloadInstruction Manual
- UOM 8Plex 7Plex 6Plex 5Plex 4Plex 3Plex 2Plex1Plex
- FOB
US$ 415
US$ 431
US$ 455
US$ 487
US$ 519
US$ 567
US$ 638
US$ 798
Add to Price Calculator
Result
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Tenascin C (TNC) ,etc. by FLIA (Flow Luminescence Immunoassay).
No significant cross-reactivity or interference between Tenascin C (TNC) ,etc. by FLIA (Flow Luminescence Immunoassay) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Tenascin C (TNC) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Tenascin C (TNC) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 85-93 | 89 |
| EDTA plasma(n=5) | 81-92 | 84 |
| heparin plasma(n=5) | 93-105 | 97 |
| sodium citrate plasma(n=5) | 80-94 | 85 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Tenascin C (TNC) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Tenascin C (TNC) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Tenascin C (TNC) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 87-105% | 91-99% | 87-94% | 92-101% |
| EDTA plasma(n=5) | 93-104% | 91-98% | 83-101% | 93-101% |
| heparin plasma(n=5) | 84-103% | 92-101% | 96-104% | 98-105% |
| sodium citrate plasma(n=5) | 78-101% | 81-95% | 78-95% | 88-103% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:TNC) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
GIVEAWAYS
INCREMENT SERVICES
| Magazine | Citations |
| Medical Principles and Practice | An investigation of tenascin-C levels in rheumatic mitral stenosis and their response to percutaneous mitral balloon valvuloplasty Pubmed: 22889719 |
| Tumor Biology | Clinical significance of serum tenascin-c levels in epithelial ovarian cancer Springer: Source |
| Tumor Biology | Clinical significance of serum tenascin-C levels in breast cancer Springer: Source |
| BioFactors | Tenascin-C is increased in atherothrombotic stroke patients and has an anti-inflammatory effect in the human carotid artery. Pubmed:24823872 |
| Connective Tissue Research | Metabolic and cytoprotective effects of in vivo peri-patellar hyaluronic acid injections in cultured tenocytes Pubmed:25333747 |
| K | Role of Tenascin-C in the differential diagnosis of small round blue cell tumors Index.Php: Sdutfd |
| Life Sci | A new predictor of mortality in hemodialysis patients; Tenascin-C PubMed: 26390818 |
| Heart Research | Biomarkers Score for Patients with Mitral Stenosis: A useful conjunction with Wilkins’s Score for Early Intervention Heartresearchopenjournal: Hroj5 |
| Araştırmalar / Researches | Pankreas Adenokarsinomunda Serum Tenascin-C Düzeyi Potansiyel Bir Biyobelirteç Midir? Cpdf:5C21620161255 |
| Archives of Dermatological Research | Increased tenascin C and DKK1 in vitiligo: possible role of fibroblasts in acral and non-acral disease Pubmed:29605863 |
| Methods in Cell Biology | How to detect and purify tenascin-C Pubmed:29310788 |
| Scientific Reports | Altered transcriptional regulatory proteins in glioblastoma and YBX1 as a potential regulator of tumor invasion Pubmed: 31358880 |
| Catalog No. | Related products for research use of Homo sapiens (Human) Organism species | Applications (RESEARCH USE ONLY!) |
| RPB975Hu02 | Recombinant Tenascin C (TNC) | Positive Control; Immunogen; SDS-PAGE; WB. |
| RPB975Hu01 | Recombinant Tenascin C (TNC) | Positive Control; Immunogen; SDS-PAGE; WB. |
| PAB975Hu01 | Polyclonal Antibody to Tenascin C (TNC) | WB; IHC |
| PAB975Hu02 | Polyclonal Antibody to Tenascin C (TNC) | WB; IHC; ICC; IP. |
| LAB975Hu71 | Biotin-Linked Polyclonal Antibody to Tenascin C (TNC) | WB; IHC; ICC. |
| MAB975Hu22 | Monoclonal Antibody to Tenascin C (TNC) | IHC |
| MAB975Hu21 | Monoclonal Antibody to Tenascin C (TNC) | IHC |
| MAB975Hu23 | Monoclonal Antibody to Tenascin C (TNC) | IHC |
| MAB975Hu24 | Monoclonal Antibody to Tenascin C (TNC) | IHC |
| SEB975Hu | ELISA Kit for Tenascin C (TNC) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| LMB975Hu | Multiplex Assay Kit for Tenascin C (TNC) ,etc. by FLIA (Flow Luminescence Immunoassay) | FLIA Kit for Antigen Detection. |