Targeting ferroptosis for liver disease
Ferroptosis, a regulated program of cell death, results from the mis-controlled accumulation of membrane lipid peroxidation in an iron-dependent manner, which is distinguished from other programmed cell deaths such as apoptosis, necrosis or pyroptosis. Factors including the depletion of glutathione (GSH), and the inhibition of cystine/glutamate antiporter system or glutathione peroxidase 4 (GPX4) can trigger ferroptosis. On the other hand, ferroptosis can be inhibited by iron chelators (e.g., deferoxamine), and lipophilic anti-oxidants (e.g., N-acetyl-L-cysteine, ferrostatin-1, and vitamin E). Notably, ferroptosis is closely related to the development and control of various liver diseases, such as steatohepatitis, fibrosis and hepatocellular carcinoma (HCC). Triggering ferroptosis is emerging as a novel and promising therapeutic approach for liver disease.
1. Celastrol induces ferroptosis to ameliorate hepatic fibrosis via targeting peroxiredoxins and HO-1
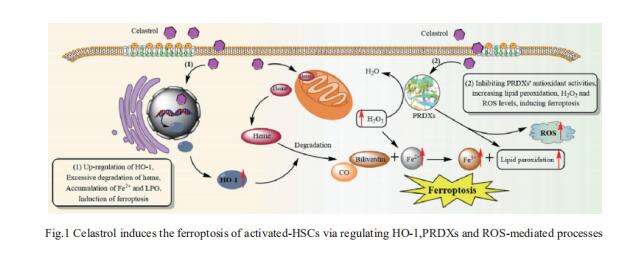
Celastrol, a natural bioactive triterpenoid extracted from Tripterygium wilfordii, shows effective anti-fibrotic and anti-inflammatory activities in multiple hepatic diseases. Jigang Wang, Department of Urology, the Second Clinical Medical College, Jinan University, China, and his team discovered that celastrol exerts anti-fibrotic effects via promoting the production of reactive oxygen species (ROS) and inducing ferroptosis in activated hepatic stellate cells (HSCs)[1]. By using activity-based protein profiling (ABPP) in combination with bio-orthogonal click chemistry reaction and cellular thermal shift assay (CETSA), they showed that celastrol directly binds to peroxiredoxins (PRDXs), including PRDX1, PRDX2, PRDX4 and PRDX6, through the active cysteine sites, and inhibits their anti-oxidant activities. Celastrol also targets to heme oxygenase 1 (HO-1) and upregulates its expression in activated-HSCs. Knockdown of PRDX1, PRDX2, PRDX4, PRDX6 or HO-1 in HSCs, to varying extent, elevated cellular ROS levels and induced ferroptosis(Fig.1). These results showed celastrol can ameliorate hepatic fibrosis via targeting PRDXs and HO-1 to induce the ferroptosis of activated-HSCs, providing a promising therapeutic strategy for hepatic fibrosis.
2. COMMD10 inhibits HIF1a/CP loop to enhance ferroptosis and radiosensitivity by disrupting Cu-Fe balance in hepatocellular carcinoma
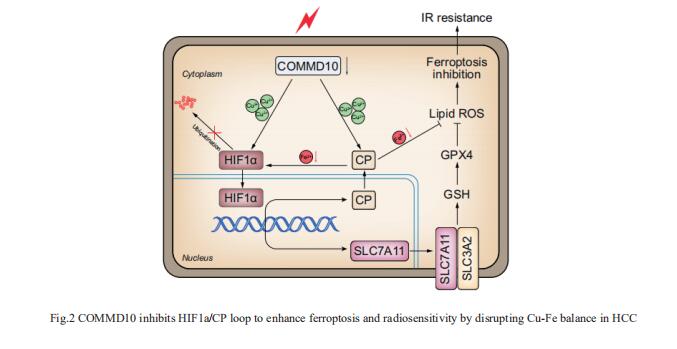
Copper (Cu) is an essential trace element whose serum levels have been reported to act as an effective indicator of the efficacy of radiotherapy. Jian Guan, Department of Radiation Oncology, Nanfang Hospital, Southern Medical University, China, and his team aimed to investigate the precise mechanism by which Cu or Cu-related proteins regulate the radiosensitivity of hepatocellular carcinoma (HCC)[2]. Ionizing radiation (IR) induced a reduction of COMMD10, which increased intracellular Cu and led to radioresistance of HCC. COMMD10 enhanced ferroptosis and radiosensitivity in vitro and in vivo. Mechanistically, low expression of COMMD10 induced by IR inhibited the ubiquitin degradation of HIF1a (by inducing Cu accumulation) and simultaneously impaired its combination with HIF1a, promoting HIF1a nuclear translocation and the transcription of ceruloplasmin (CP) and SLC7A11, which jointly inhibited ferroptosis in HCC cells (Fig.2). In addition, elevated CP promoted HIF1a expression by reducing Fe, forming a positive feedback loop. COMMD10 inhibits the HIF1a/CP loop to enhance ferroptosis and radiosensitivity by disrupting Cu-Fe homeostasis in HCC. This work provides new targets and treatment strategies for overcoming radioresistance in HCC.
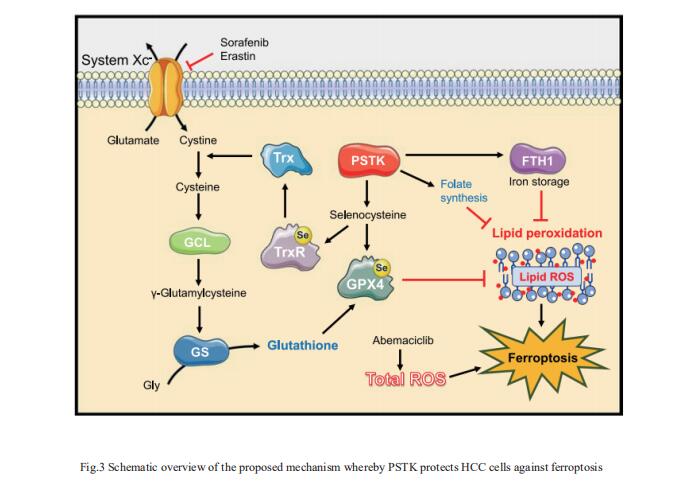
3. PSTK as a regulator of chemotherapy-induced ferroptosis in HCC
The emergence of therapeutic resistance has hampered the efficacy of targeted treatments employed to treat HCC patients to date. Jun Liang, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Oncology, Peking University Cancer Hospital and Institute, China, and his team conducted a series of CRISPR/Cas9 screens to identify genes associated with synthetic lethality capable of improving HCC patient clinical responses[3]. The inhibition of phosphoseryl-tRNA kinase (PSTK) was found to increase HCC cell sensitivity to chemotherapeutic treatment. PSTK was associated with the suppression of chemotherapy-induced ferroptosis in HCC cells, and the depletion of PSTK resulted in the inactivation of GPX4 and the disruption of GSH metabolism owing to the inhibition of selenocysteine and cysteine synthesis, thus enhancing the induction of ferroptosis upon targeted chemotherapeutic treatment (Fig.3). Punicalin was identified as a possible PSTK inhibitor that exhibited synergistic efficacy when applied together with Sorafenib to treat HCC in vitro and in vivo. The study is the first to have identified PSTK as a critical mediator of ferroptosis resistance in HCC cells, inhibiting PSTK may represent a novel and viable approach to overcoming targeted therapy resistance in HCC patients.
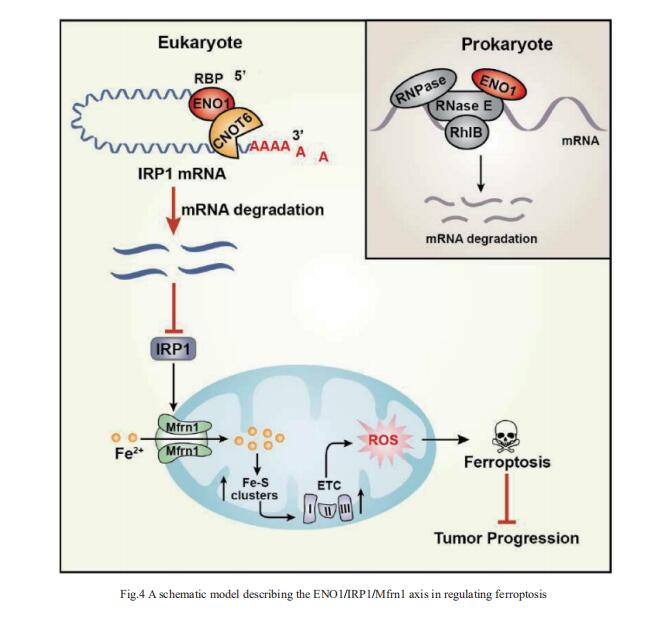
4. ENO1 suppresses cancer cell ferroptosis by degrading the mRNA of iron regulatory protein 1
α-Enolase 1 (ENO1) is a critical glycolytic enzyme whose aberrant expression drives the pathogenesis of various cancers. Huafeng Zhang, Division of Life Sciences and Medicine, University of Science and Technology of China, China, and his team showed that ENO1 suppresses iron regulatory protein 1 (IRP1) expression to regulate iron homeostasis and survival of hepatocellular carcinoma (HCC) cells[4]. Mechanistically, ENO1 recruits CNOT6 to accelerate the mRNA decay of IRP1 in cancer cells, leading to inhibition of mitoferin-1 (Mfrn1) expression and subsequent repression of mitochondrial iron-induced ferroptosis(Fig.4). Moreover, through in vitro and in vivo experiments and clinical sample analysis, we identified IRP1 and Mfrn1 as tumor suppressors by inducing ferroptosis in HCC cells. This study establishes a novel role for the ENO1/IRP1/Mfrn1 pathway in the pathogenesis of HCC and reveals a previously unknown connection between the ENO1/IRP1/Mfrn1 pathway and ferroptosis, suggesting a potential innovative cancer therapy.
References
[1]Luo P, Liu D, Zhang Q, et al. Celastrol induces ferroptosis in activated HSCs to ameliorate hepatic fibrosis via targeting peroxiredoxins and HO-1 [J]. Acta Pharm Sin B. 2022,12(5):2300-2314. (IF=14.903)
[2]Yang M, Wu X, Hu J, et al. COMMD10 inhibits HIF1α/CP loop to enhance ferroptosis and radiosensitivity by disrupting Cu-Fe balance in hepatocellular carcinoma [J]. J Hepatol. 2022,76(5):1138-1150. (IF=30.083)
[3]Chen Y, Li L, Lan J, et al. CRISPR screens uncover protective effect of PSTK as a regulator of chemotherapy-induced ferroptosis in hepatocellular carcinoma [J]. Mol Cancer. 2022,21(1):11. (IF=41.444)
[4]Zhang T, Sun L, Hao Y, et al. ENO1 suppresses cancer cell ferroptosis by degrading the mRNA of iron regulatory protein 1 [J]. Nat Cancer. 2022,3(1):75-89. (IF=23.177)
Cloud-Clone not only provides animal models of various liver diseases, including liver fibrosis, liver ischemia, alcoholic liver disease, intrahepatic cholestasis, liver cirrhosis, fatty liver, liver failure, etc., covering common liver diseases. We also have a variety of commonly used detection indicators for liver diseases and ferroptosis-related protein detection products, which can help scientific researchers to conduct research on liver diseases and ferroptosis.