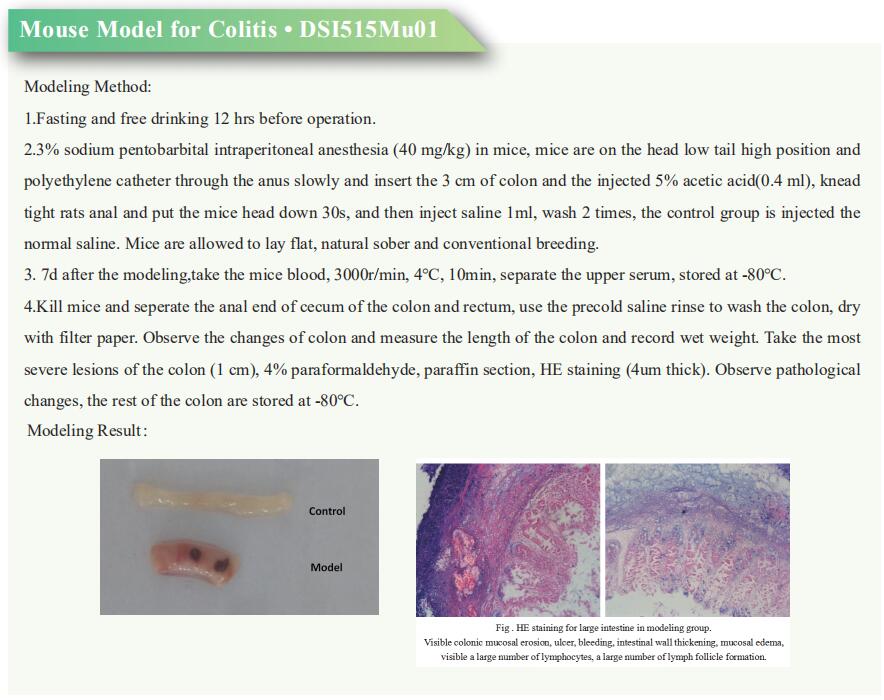
Research progress of irritable bowel syndrome
Irritable bowel syndrome (IBS) is a common medical condition characterized by chronic, recurrent, abdominal pain and discomfort, and altered bowel habits that occur in the absence of other organic gastrointestinal (GI) disease. IBS generates a significant health care burden and can severely impair quality of life. Altered gastrointestinal motility, visceral hypersensitivity, post infectious reactivity, brain-gut interactions, alteration in fecal micro flora, bacterial overgrowth, food sensitivity, carbohydrate malabsorption, and intestinal inflammation all have been implicated in the pathogenesis of IBS. A better understanding of the pathogenesis of IBS may help to develop new treatments to improve the quality of life of patients.
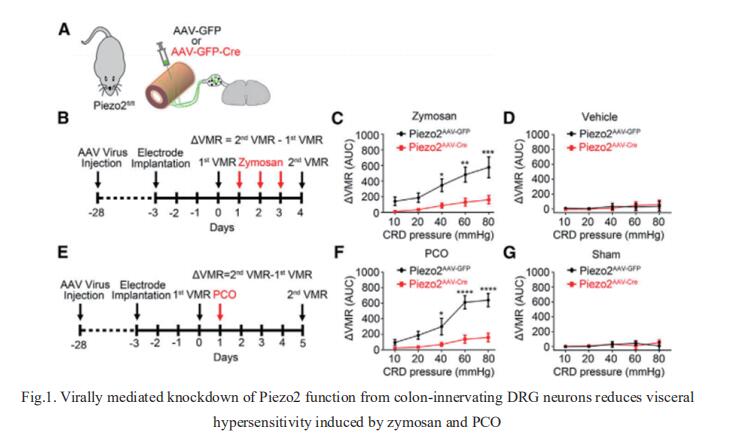
1. Piezo2 channels expressed by colon-innervating TRPV1-lineage neurons mediate visceral mechanical hypersensitivity
Inflammatory and functional gastrointestinal disorders such as irritable bowel syndrome (IBS) and obstructive bowel disorder (OBD) underlie the most prevalent forms of visceral pain. Although visceral pain can be generally provoked by mechanical distension/stretch, the mechanisms that underlie visceral mechanosensitivity in colon-innervating visceral afferents remain elusive. Hongzhen Hu, Department of Anesthesiology, The Center for the Study of Itch & Sensory Disorders, Washington University School of Medicine, USA, and his team showed that virally mediated ablation of colon-innervating TRPV1-expressing nociceptors markedly reduces colorectal distention (CRD)-evoked visceromotor response (VMR) in mice[1]. Selective ablation of the stretch-activated Piezo2 channels from TRPV1 lineage neurons substantially reduces mechanically evoked visceral afferent action potential firing and CRD-induced VMR under physiological conditions, as well as in mouse models of zymosan-induced IBS and partial colon obstruction (PCO) (Fig.1). Collectively, their results demonstrate that mechanosensitive Piezo2 channels expressed by TRPV1-lineage nociceptors powerfully contribute to visceral mechanosensitivity and nociception under physiological conditions and visceral hypersensitivity under pathological conditions in mice, uncovering potential therapeutic targets for the treatment of visceral pain.
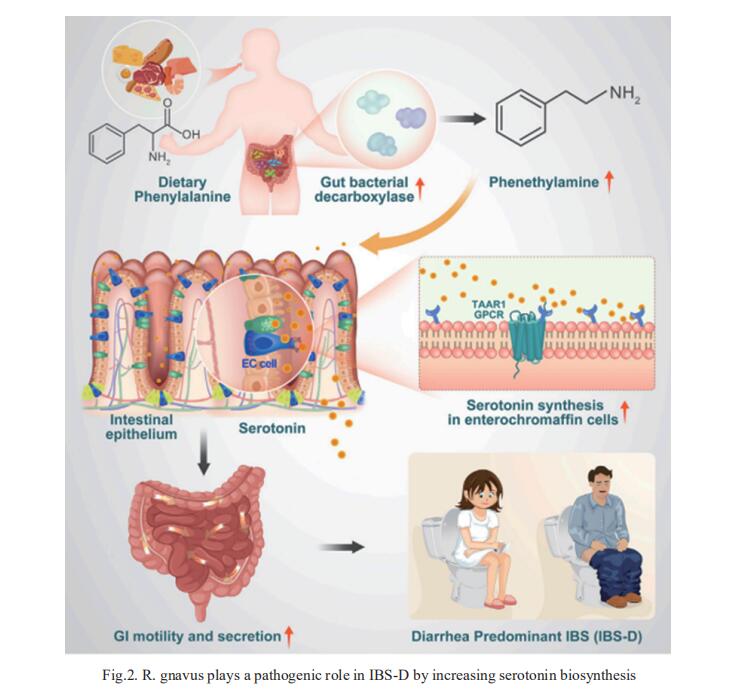
2. Ruminococcus gnavus plays a pathogenic role in diarrhea-predominant irritable bowel syndrome by increasing serotonin biosynthesis
Diarrhea-predominant irritable bowel syndrome (IBS-D) is associated with elevated serotonin that increases gut motility. Zhao-xiang Bian, Centre for Chinese Herbal Medicine Drug Development Limited, Hong Kong Baptist University, China, and his team determined that the bacterium Ruminococcus gnavus plays a pathogenic role in IBS-D[2]. Monocolonization of germ-free mice with R. gnavus induced IBS-D-like symptoms, including increased GI transit and colonic secretion, by stimulating the production of peripheral serotonin. R. gnavus-mediated catabolism of dietary phenylalanine and tryptophan generated phenethylamine and tryptamine that directly stimulated serotonin biosynthesis in intestinal enterochromaffin cells via a mechanism involving activation of trace amine-associated receptor 1 (TAAR1) (Fig.2). This R. gnavus-driven increase in serotonin levels elevated GI transit and colonic secretion but was abrogated upon TAAR1 inhibition. Collectively, this study provides molecular and pathogenetic insights into how gut microbial metabolites derived from dietary essential amino acids affect serotonin-dependent control of gut motility.
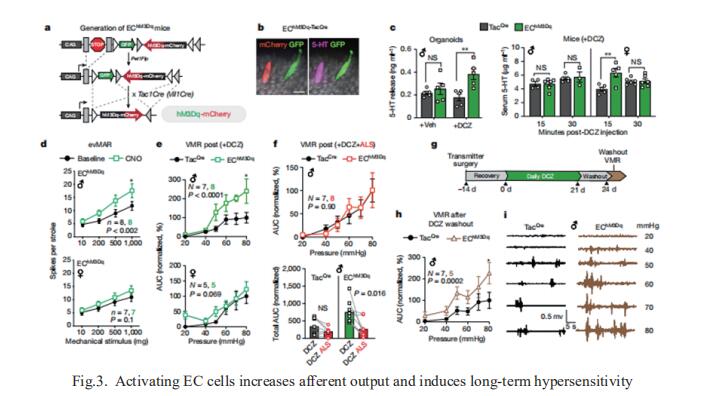
3. Gut enterochromaffin cells drive visceral pain and anxiety
For the growing population afflicted by IBS, gastrointestinal hypersensitivity and pain persist long after tissue injury has resolved. David Julius, Department of Physiology, University of California, USA, and his team focused on enterochromaffin (EC) cells, which are rare excitable, serotonergic neuroendocrine cells in the gut epithelium[3]. By enhancing or suppressing EC cell function in vivo, they showed that these cells are sufficient to elicit hypersensitivity to gut distension and necessary for the sensitizing actions of isovalerate, a bacterial short-chain fatty acid associated with gastrointestinal inflammation. Remarkably, prolonged EC cell activation produced persistent visceral hypersensitivity, even in the absence of an instigating inflammatory episode (Fig.3). Furthermore, perturbing EC cell activity promoted anxiety-like behaviours which normalized after blockade of serotonergic signalling. Their findings validate a critical role for EC cell-mucosal afferent signalling in acute and persistent gastrointestinal pain.
References
[1]Xie Z, Feng J, Hibberd TJ, et al. Piezo2 channels expressed by colon-innervating TRPV1-lineage neurons mediate visceral mechanical hypersensitivity. Neuron. 2023;111(4):526-538.e4. (IF=18.688)
[2]Zhai L, Huang C, Ning Z, et al. Ruminococcus gnavus plays a pathogenic role in diarrhea-predominant irritable bowel syndrome by increasing serotonin biosynthesis. Cell Host Microbe. 2023;31(1):33-44.e5. (IF=31.316)
[3]Bayrer JR, Castro J, Venkataraman A, et al. Gut enterochromaffin cells drive visceral pain and anxiety. Nature. 2023;616(7955):137-142. (IF=69.504)
Cloud-Clone not only provides animal models of various digestive system diseases, including irritable bowel syndrome, Crohn's disease, ulcerative colitis, gastritis, duodenal ulcer and other common digestive disease animal models. We also have various digestive system diseases detection indicators and the above TRPV1, TAAR, 5-HT and other related products, which can help the vast number of researchers to carry out digestive system diseases related research.