Progress in the research of disease mechanism and potential treatment of Alzheimer's disease
Alzheimer’s disease (AD), the most common cause of agerelated dementia, represents one of the most urgent healthcare challenges facing us today with numbers affected projected to increase 50 million worldwide to 75 million by 2030 in line with an aging global population. It is pathologically characterized by abnormal accumulation of amyloid β (Aβ) as amyloid plaques, hyperphosphorylated tau in the form of neurofibrillary tangles, and neuroinflammation in the brain.
Research progress in the pathogenesis and potential treatment of AD
In the past several decades, many studies have been carried out to investigate cerebrospinal fluid biomarkers (tau and Aβ), neuroimaging measurements of hippocampal/cortical atrophy and Aβ deposition in the brain, mitochondrial disturbance, and microglial dysfunction and immune response in AD to better understand metabolic alterations in the early stage of AD. Recently, new insights into AD disease have been reported in a number of high impact factors paper, which may serve as a potential approach for targeted treatment of AD.
1. Deletion of Abi3 gene locus exacerbates neuropathological features of AD in a mouse model of Aβ amyloidosis
Studies have shown that Abi3 gene variants increase the risk of late onset AD. Since the physiological role of Abi3 in the brain, especially in microglia, and the mechanisms contributing to the etiology of AD are not well understood, Jungsu Kim of Indiana University School of Medicine and his team investigated the role of Abi3 in the pathological features of AD by using the 5XFAD mouse model[1]. They found that deletion of Abi3 locus significantly increased Aβlevels(Fig.1) and amyloid plaques and decreased microglia clustering around the plaques. Mechanism studies suggested that Abi3 knockdown in microglia impairing migration and phagocytosis. Given the functional data shown in this study and its significant genetic association with AD, Abi3 and its downstream pathways may be promising therapeutic targets.
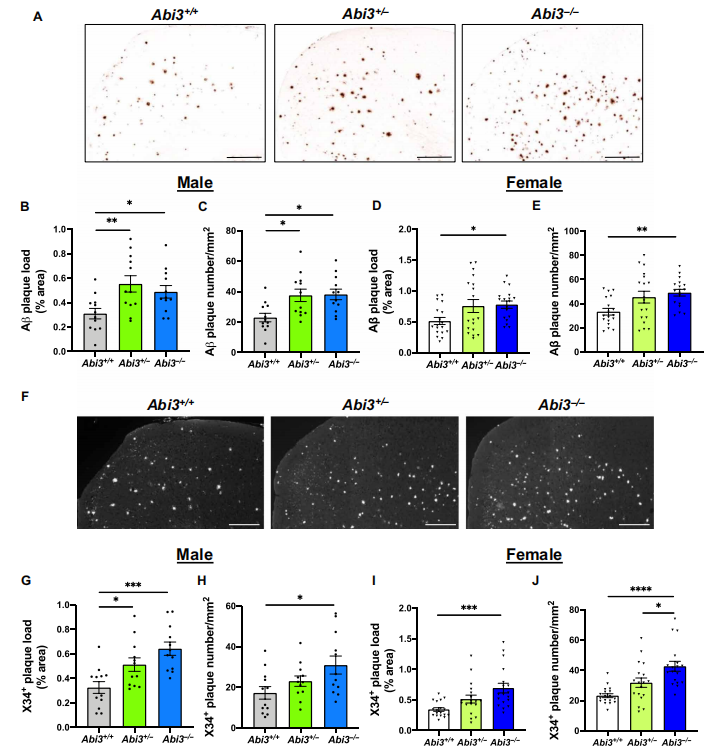
Fig.1 Deletion of Abi3 gene locus increases amyloid plaques in 5XFAD mouse cortices
2. Muscarinic M1 receptor agonist with the potential to treat AD
Based on its high expression in areas such as the hippocampus and cortex and the pro-cognitive effects in pre-clinical animal studies, the M1 muscarinic acetylcholine receptor (M1-receptor) is widely considered to be a key or mediator of cognitive function and thereby a target for the treatment in AD. Andrew B. Tobin of University of Glasgow and his team aimed to design the drug properties needed for a well-tolerated M1-agonist with the potential to alleviate cognitive loss by taking a stepwise translational approach from atomic structure, cell/tissue-based assays, evaluation in preclinical species, clinical safety testing, and finally establishing activity in memory centers in humans(Fig.2)[2]. Through this approach, they rationally designed the optimal properties, including selectivity and partial agonism, into HTL9936—a potential candidate for the treatment of memory loss in AD.
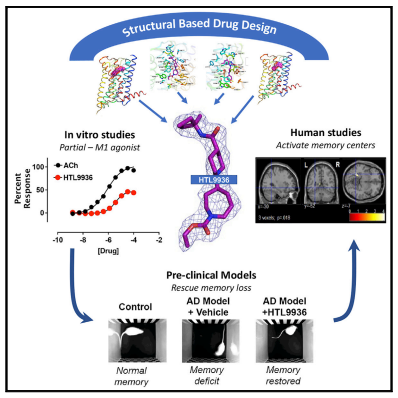
Fig.2 Design of a muscarinic M1 receptor agonist
3. Integrative metabolomics-genomics approach reveals key metabolic pathways and regulators of AD
Metabolites, the biochemical products of the cellular process, can be used to measure alterations in biochemical pathways related to the pathogenesis of AD. Bin Zhang of Icahn School of Medicine at Mount Sinai and his team aim to identify AD specific metabolomic changes and their potential upstream genetic and transcriptional regulators through an integrative systems biology framework for analyzing genetic, transcriptomic, metabolomic, and proteomic data in AD(Fig.3)[3]. They found short-chain acylcarnitines/amino acids and medium/long-chain acylcarnitines are most associated with AD clinical outcomes. Integration of the gene expression data showed ABCA1 and CPT1A are involved in the regulation of acylcarnitines and amino acids in AD. Increased ABCA1 gene expression and adiponectin protein, a regulator of ABCA1, correspond to decreased short-chain acylcarnitines and amines in AD. These findings may help to develop sensitive and specific biomarkers for the diagnosis of AD and identify new molecular mechanisms for the pathogenesis of AD.
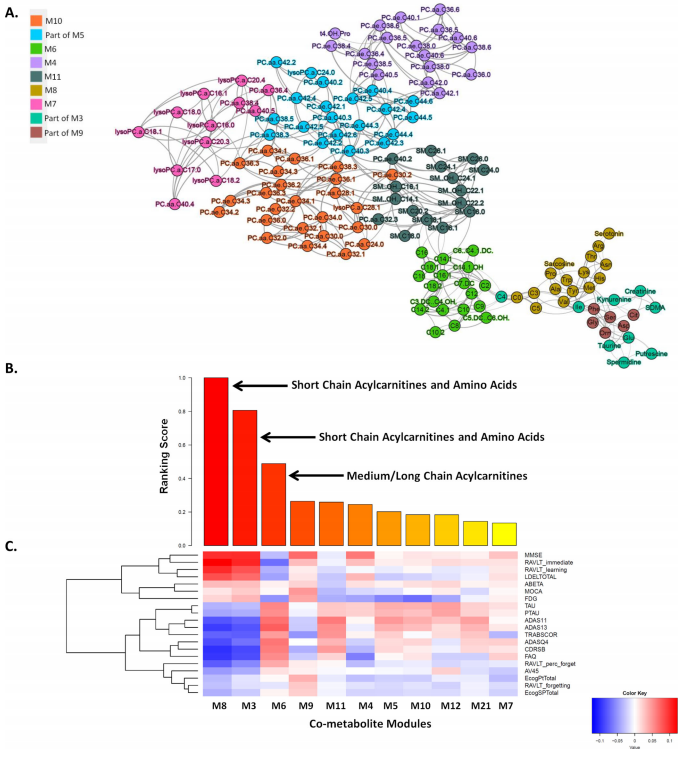
Fig.3 Multiscale metabolite co-expression network analysis of the blood metabolomic data in AD
4. Restoring activity in the thalamic reticular nucleus improves sleep architecture and reduces Aβaccumulation
Sleep disruptions promote increases of Aβ and tau in the brain and increase AD risk. The thalamic reticular nucleus (TRN) is essential for sleep maintenance and for the regulation of slow-wave sleep (SWS). Jeannie Chin of Memory and Brain Research Center, Baylor College of Medicine, and his team examined the TRN in transgenic mice that express mutant human amyloid precursor protein (APP) and found reduced neuronal activity, increased sleep fragmentation, and decreased SWS time as compared to nontransgenic littermates[4]. Selective activation of the TRN using excitatory DREADDs restored sleep maintenance, increased time in SWS, and reduced amyloid plaque load in both hippocampus and cortex(Fig.4). These findings suggest that the TRN may play a major role in symptoms associated with AD. Enhancing TRN activity might be a promising therapeutic strategy for AD.
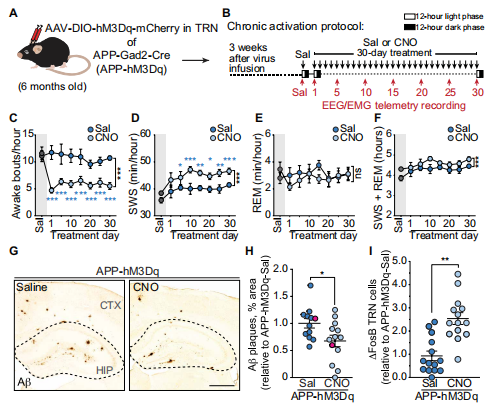
Fig.4 Effect of chronic activation of the TRN on sleep and amyloid plaque deposition
References
[1] Karahan H, Smith DC, Kim B, et al. Deletion of Abi3 gene locus exacerbates neuropathological features of Alzheimer's disease in a mouse model of Aβ amyloidosis [J]. Sci Adv. 2021, 7(45):eabe3954. (IF=14.136)
[2] Brown AJH, Bradley SJ, Marshall FH, et al. From structure to clinic: Design of a muscarinic M1 receptor agonist with potential to treatment of Alzheimer's disease [J]. Cell. 2021;184(24):5886-5901. (IF=41.582)
[3] Horgusluoglu E, Neff R, Song WM, et al. Integrative metabolomics-genomics approach reveals key metabolic pathways and regulators of Alzheimer's disease [J]. Alzheimers Dement. 2021, 10.1002/alz.12468. (IF=21.566)
[4] Jagirdar R, Fu CH, Park J, et al. Restoring activity in the thalamic reticular nucleus improves sleep architecture and reduces Aβ accumulation in mice [J]. Sci Transl Med. 2021, 13(618):eabh4284. (IF=17.956)
Cloud-Clone can not only provide animal models of various neurological diseases, including Alzheimer's disease, Parkinson's disease, anxiety disorder, chronic stress depression, etc., cover common neurological diseases. It also has various neurological disease detection indicators related products, which can help the majority of scientific researchers to carry out neurological disease related research.