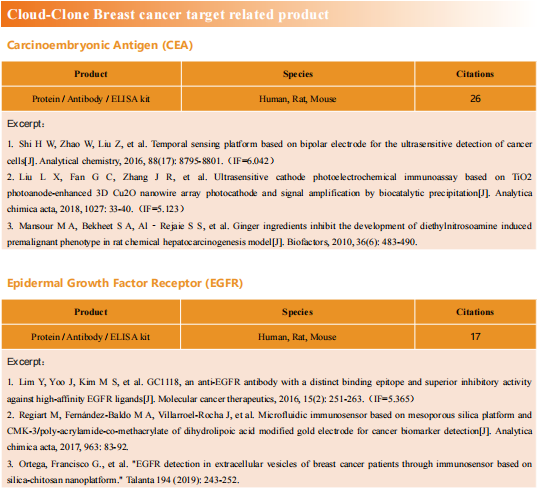
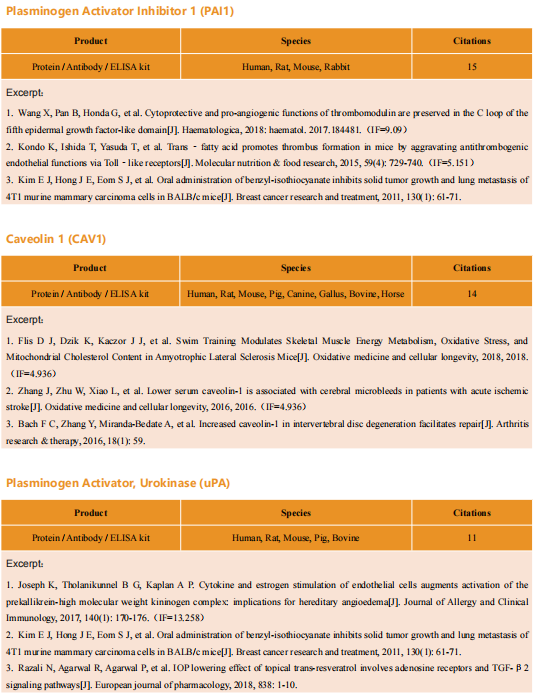
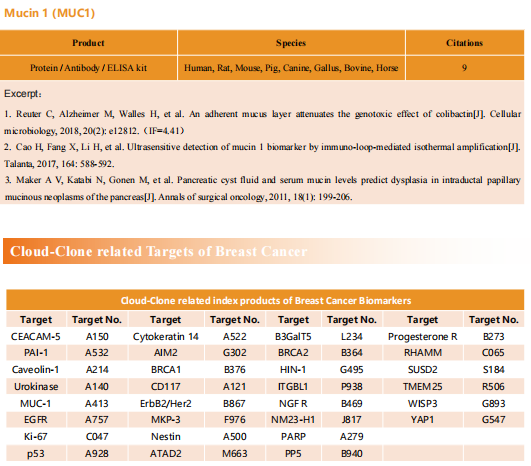
New strategies for triple-negative breast cancer treatment
Breast cancer (BC) is the most common malignancy in women. Due to advances in early diagnosis and comprehensive treatment strategies, the prognosis of breast cancer patients has improved. The disease has been divided into different subtypes, largely on the basis of the presence or absence of three key proteins: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2/ERBB2). Among them, triple negative breast cancer (TNBC) with negative expression of ER, PR and HER2/ERBB2 is the most difficult type of breast cancer treatment at present.
New treatment strategies for TNBC
TNBC has been characterized by several aggressive clinical features including high rates of metastasis, recurrence, poor survival and high breast tumor initiating cell population compared to other BC subtypes. Due to lacking of specific therapeutic targets, it appears to be extremely urgent to find a reliable and effective strategy for TNBC. The occurrence rate of metastasis is also increased, and approximately 90% of cancer-related deaths are attributed to metastasis. Therefore, systematic and deepened research on the molecular heterogeneity of TNBC, would afford the ability to explore more effective metastasis-targeting agents and improve the prognostication of patients.
1. Fatty acid oxidation is a druggable gateway regulating cellular plasticity for driving metastasis in breast cancer
Epithelial-to-mesenchymal transition (EMT) confers cancer stem cell-like properties, enhanced tumorigenicity and drug resistance to tumor cells, while mesenchymal-epithelial transition (MET) reverses these phenotypes. By using high-throughput chemical library screens, Wai Leong Tam's team from the Genome Institute of Singapore found retinoids are potent promoters of MET that inhibit tumorigenicity in basal-like breast cancer[1]. Retinoids bind cognate nuclear receptors, which target lipid metabolism genes, thereby redirecting fatty acids for β-oxidation in the mesenchymal cell state towards lipid storage in the epithelial cell state(Fig 1). Disruptions of key metabolic enzymes mediating this flux inhibit MET. Conversely, perturbations to fatty acid oxidation rechannel fatty acid flux and promote a more epithelial cell phenotype, blocking EMT-driven breast cancer metastasis in animal models. The addiction to specific metabolic pathways during distinct steps of cancer progression may be exploited to target specific cell states and opens up new avenues for antimetabolite therapeutic strategies.
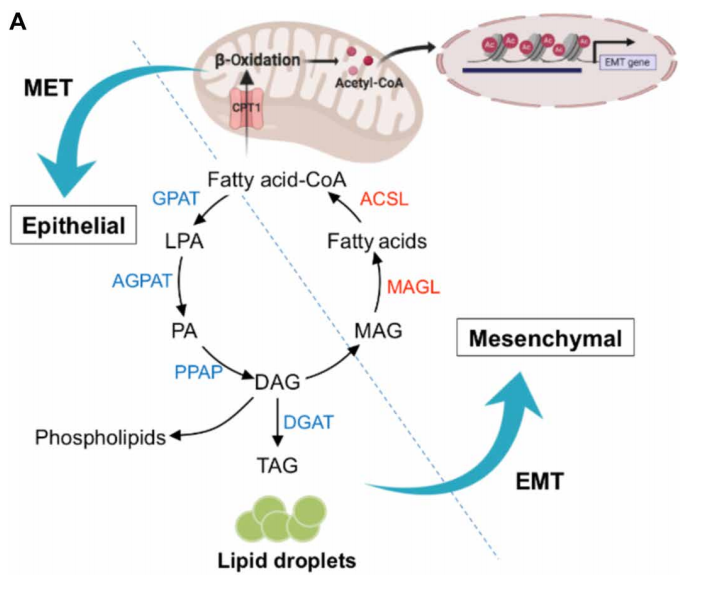
Fig 1. Rewiring of fatty acid metabolism supports cell state transitions
2. Targeting neurons in the tumor microenvironment with bupivacaine nanoparticles reduces breast cancer progression and metastases
Neurons within the tumor microenvironment promote cancer progression; thus, their local targeting has potential clinical benefits. Avi Schroeder's team from the Department of Chemical Engineering at the Technion-Israel Institute of Technology designed PEGylated lipid nanoparticles loaded with a non-opioid analgesic, bupivacaine, to target neurons within breast cancer tumors and suppress nerve-to-cancer cross-talk[2]. They demonstrated that signaling between TNBC cells (4T1) and neurons involves secretion of cytokines stimulating neurite outgrowth. Reciprocally, neurons stimulated 4T1 proliferation, migration, and survival through secretion of neurotransmitters. Bupivacaine curbs neurite growth and signaling with cancer cells, inhibiting cancer cell viability(Fig 2). In vivo, bupivacaine-loaded nanoparticles intravenously administered suppressed neurons in orthotopic TNBC tumors, inhibiting tumor growth and metastatic dissemination. This study suggests that targeting nerves in the tumor tissue using non-opioid anesthetic nanoparticles is a potential new clinical approach that can improve TNBC therapy.
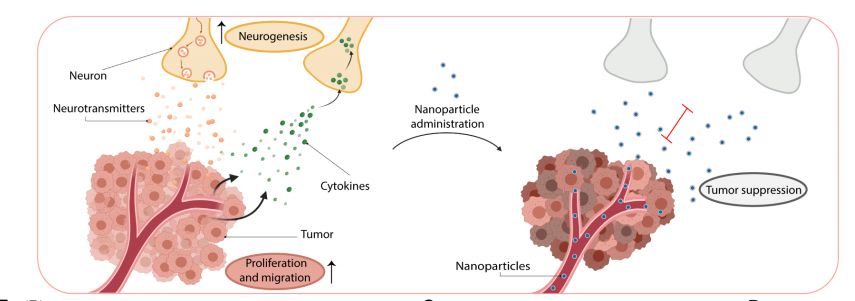
Fig 2. using lipid nanoparticles loaded with bupivacaine to curb nerve/cancer cross-talk and inhibit tumor growth
3. ALDH1A1 activity in tumor-initiating cells remodels myeloid-derived suppressor cells to promote breast cancer progression
Tumor-initiating cells (TIC) are associated with tumor initiation, growth, metastasis, and recurrence. Aldehyde dehydrogenase 1A1 (ALDH1A1) is a TIC marker in many cancers. Suling Liu and his team at Fudan University Shanghai Cancer Center demonstrated that ALDH1A1 enzymatic activity facilitates breast tumor growth[3](Fig 3). Mechanistically, ALDH1A1 decreased the intracellular pH in breast cancer cells to promote phosphorylation of TAK1, activate NFκB signaling, and increase the secretion of granulocyte macrophage colony-stimulating factor, which led to myeloid-derived suppressor cell expansion and immunosuppression. Furthermore, the ALDH1A1 inhibitor disulfiram and chemotherapeutic agent gemcitabine cooperatively inhibited breast tumor growth and tumorigenesis by purging ALDH+ TICs and activating T cell immunity. These findings elucidate how active ALDH1A1 modulates the immune system to promote tumor development, highlghting new therapeutic strategies for malignant breast cancer.
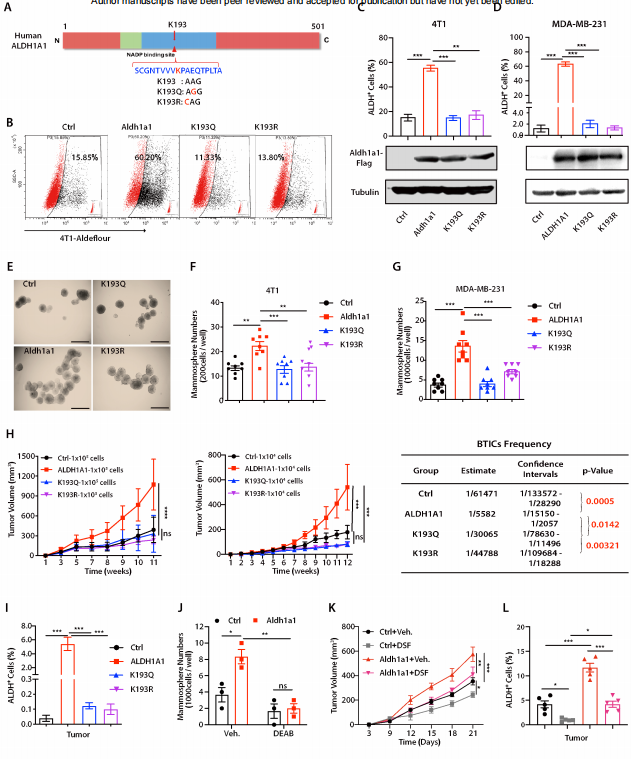
Fig 3. ALDH1A1 increased ALDH+ TICs and promoted breast tumor development relying on enzyme activity
References
[1] Loo SY, Toh LP, Xie WH, et al. Fatty acid oxidation is a druggable gateway regulating cellular plasticity for driving metastasis in breast cancer[J]. Sci Adv, 2021, 7(41): eabh2443. (IF=14.136)
[2] Kaduri M, Sela M, Kagan S, et al. Targeting neurons in the tumor microenvironment with bupivacaine nanoparticles reduces breast cancer progression and metastases[J]. Sci Adv, 2021, 7(41): eabj5435. (IF=14.136)
[3] Liu C, Qiang J, Deng Q, et al. ALDH1A1 activity in tumor-initiating cells remodels myeloid-derived suppressor cells to promote breast cancer progression.[J] .Cancer Res, 2021, Sep 27;canres.1337. (IF=12.701)
Cloud-Clone provide a variety of tumor experimental animal models, including tumor transplantation animal models, spontaneous tumor animal models, induced tumor animal models, tumor metastasis animal models, etc., covering common tumor research. And we also has various cancer detection indicators and the above-mentioned ALDH1A1 pathways related products, which can help the majority of scientific researchers to carry out cancer related research.