New progress in glaucoma research
The glaucomas are a group of optic neuropathies characterized by progressive degeneration of retinal ganglion cells. Glaucoma affects more than 70 million people worldwide, making it the leading cause of irreversible blindness in the world. Glaucoma can remain asymptomatic until it is severe, resulting in a high likelihood that the number of affected individuals is much higher than the number known to have it. Glaucomas can be classified into 2 broad categories: open-angle glaucoma and angle-closure glaucoma. Both open-angle and angle-closure glaucoma can be primary diseases. Secondary glaucoma can result from trauma, certain medications such as corticosteroids, inflammation, tumor, or conditions such as pigment dispersion or pseudo-exfoliation. Currently available treatments cannot reverse glaucomatous damage to the visual system; however, early diagnosis and treatment can prevent progression of the disease. Recently, multiple studies have reported on glaucoma, which may provide assistance for the prevention and treatment of this disease.
1. Large-scale multitrait genome-wide association analyses identify hundreds of glaucoma risk loci
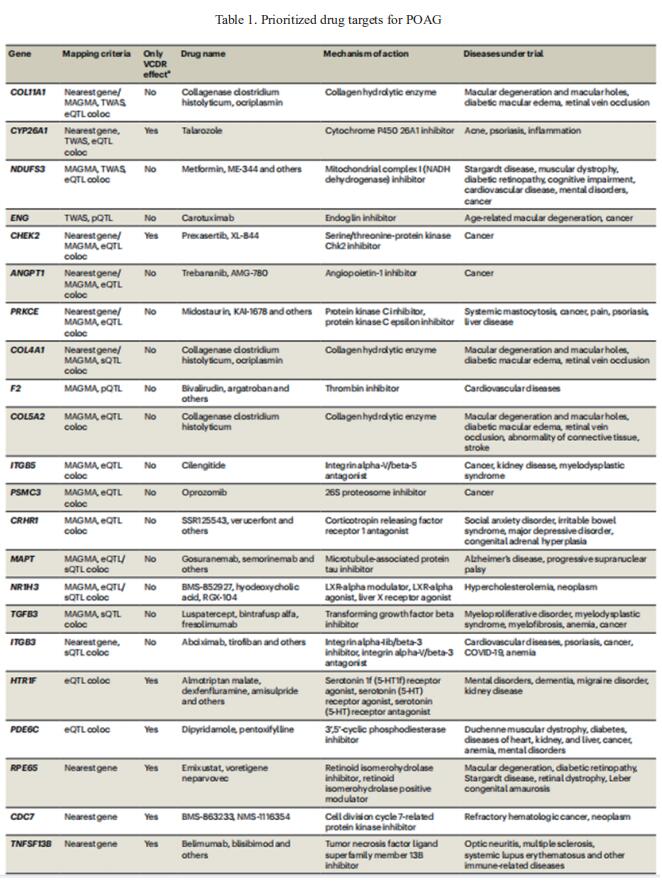
Glaucoma is a highly heritable human disease. Since much of glaucoma heritability remains unexplained, Xikun Han, Statistical Genetics Lab, QIMR Berghofer Medical Research Institute, Australia, and his team conducted a large-scale multitrait genome-wide association study in participants of European ancestry combining primary open-angle glaucoma (POAG) and its two associated traits to substantially improve genetic discovery power[1]. They further increased our power by then employing a multiancestry approach, which increased the number of independent risk loci to 312. Leveraging multiomics datasets, they identified many potential druggable genes (table 1), including neuro-protection targets likely to act via the optic nerve, a key advance for glaucoma because all existing drugs only target intraocular pressure. They also identified novel associations of POAG with other complex traits, including numerous immune-related diseases. These findings provide insights into the pathogenesis of POAG and enable new drug development for this common cause of blindness.
2. Matrix metalloproteinase-3 (MMP-3)–mediated gene therapy for glaucoma
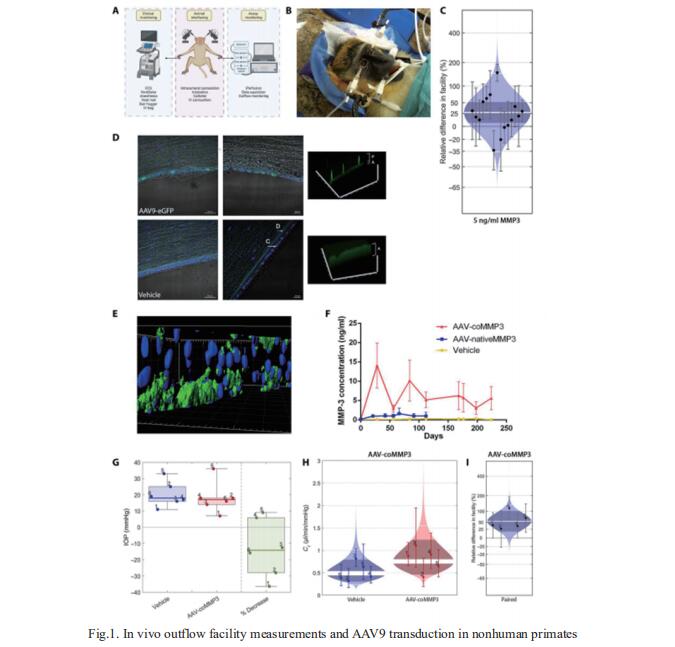
Substantial issues surrounding patient compliance remain with topical eye drops, and up to 10% of patients become treatment resistant, putting them at risk of permanent vision loss. The major risk factor for glaucoma is elevated intraocular pressure, which is regulated by the balance between the secretion of aqueous humor and the resistance to its flow across the conventional outflow pathway. Matthew Campbell, Smurfit Institute of Genetics, Trinity College Dublin, Ireland, and his team showed that adeno-associated virus 9 (AAV9)–mediated expression of matrix metalloproteinase-3 (MMP-3) can increase outflow in two murine models of glaucoma and in nonhuman primates[2]. They show that long-term AAV9 transduction of the corneal endothelium in the nonhuman primate is safe and well tolerated (Fig.1). Last, MMP-3 increases outflow in donor human eyes. Collectively, their data suggest that glaucoma can be readily treated with gene therapy–based methods, paving the way for deployment in clinical trials.
3. Pathologically high intraocular pressure disturbs normal iron homeostasis and leads to retinal ganglion cell ferroptosis in glaucoma
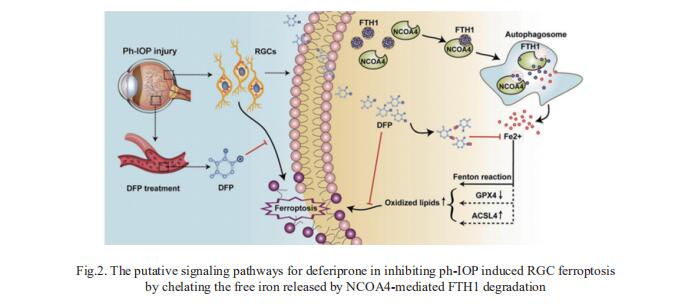
Glaucoma can result in retinal ganglion cell (RGC) death and permanently damaged vision. Pathologically high intraocular pressure (ph-IOP) is the leading cause of damaged vision during glaucoma; however, controlling ph-IOP alone does not entirely prevent the loss of glaucomatous RGCs, and the underlying mechanism remains elusive. Xiaobo Xia, Eye Center of Xiangya Hospital, Central South University, China, and his team reported an increase in ferric iron in patients with acute primary angle-closure glaucoma (the most typical glaucoma with ph-IOP damage) compared with the average population by analyzing free iron levels in peripheral serum[3]. Nuclear receptor coactivator 4 (NCOA4)-mediated degradation of ferritin heavy polypeptide 1(FTH1) is essential to disrupt iron metabolism in the retina after ph-IOP injury. Furthermore, knockdown of NCOA4 in vivo inhibited FTH1 degradation and reduced the retinal ferrous iron level. Elevated ferrous iron induced by ph-IOP led to a marked accumulation of pro-ferroptotic factors and a depletion of anti-ferroptotic factors. These biochemical changes resulted in RGC ferroptosis. Deferiprone can pass through the bloodretinal barrier after oral administration and chelated abnormally elevated ferrous iron in the retina after ph-IOP injury, thus inhibiting RGC ferroptosis and protecting visual function (Fig.2). In conclusion, this study revealed the role of NCOA4-FTH1-mediated disturbance of iron metabolism and ferroptosis in RGCs during glaucoma.
References
[1]Han X, Gharahkhani P, Hamel AR, et al. Large-scale multitrait genome-wide association analyses identify hundreds of glaucoma risk loci. Nat Genet. 2023;55(7):1116-1125. (IF=41.307)
[2]O'Callaghan J, Delaney C, O'Connor M, et al. Matrix metalloproteinase-3 (MMP-3)-mediated gene therapy for glaucoma. Sci Adv. 2023;9(16):eadf6537. (IF=12.067)
[3]Yao F, Peng J, Zhang E, et al. Pathologically high intraocular pressure disturbs normal iron homeostasis and leads to retinal ganglion cell ferroptosis in glaucoma. Cell Death Differ. 2023;30(1):69-81. (IF=12.067)
Cloud-Clone can not only provide a variety of intraocular disease animal models, including diabetic retinopathy, retinal ischemia-reperfusion injury, central retinal artery/vein occlusion, retinal detachment, ocular hypertension, ocular hypotension and other common intraocular diseases. We also have various intraocular disease detection indicators and the above-mentioned MMP-3, NCOA, FTH1 and other related products, which can help the vast number of scientific researchers to conduct research on the treatment of intraocular diseases.