New findings of CAR-T therapy in solid tumors
Chimeric antigen receptor (CAR) T cell immunotherapy refers to an adoptive immunotherapy that has rapidly developed in recent years. It is a novel type of treatment that enables T cells to express specific CARs on their surface, then returns these T cells to tumor patients to kill the corresponding tumor cells. CAR-T cell immunotherapy has demonstrated significant curative effects in the treatment of hematologic malignancies. These significant achievements promote its application in the treatment of solid tumors. In contrast with the therapeutic effect of CAR-T cell immunotherapy in hematological malignancies, the general curative effect on solid tumors is insignificant. Therefore, CAR-T cell immunotherapy has great potential in the treatment of solid tumors, providing a novel idea and method for the clinical treatment.
1. CAR-T cell killing requires the IFNγR pathway in solid tumours
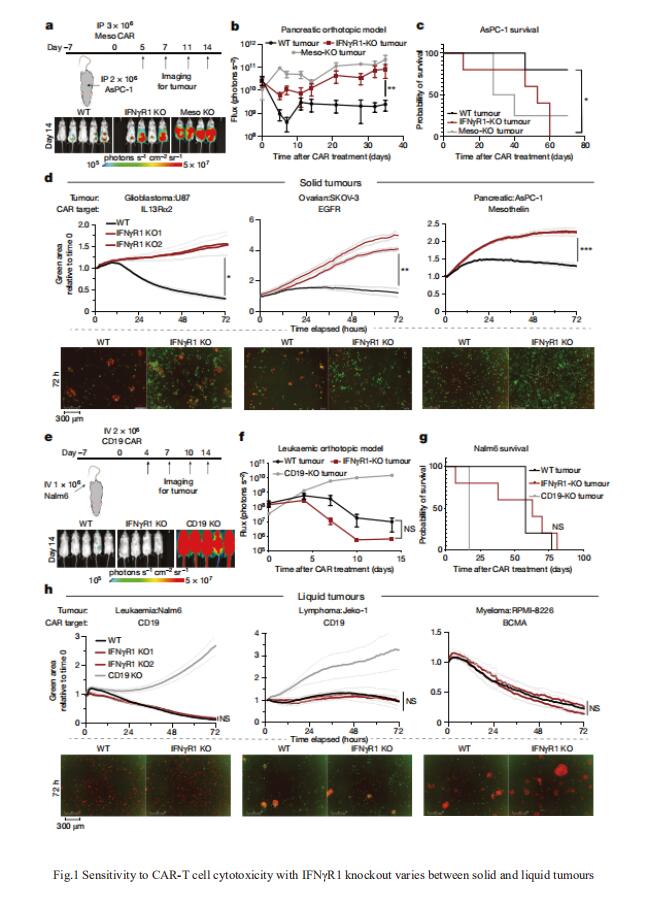
Solid tumours may have cell-intrinsic resistance mechanisms to CAR-T cell cytotoxicity. Marcela V. Maus, Cellular Immunotherapy Program, Cancer Center, Massachusetts General Hospital, USA, and his team conducted a genome-wide CRISPR knockout screen in glioblastoma, a disease in which CAR-T cells have had limited efficacy, to systematically identify potential resistance pathways in an unbiased manner[1]. They found that the loss of genes in the interferon-γ receptor (IFNγR) signalling pathway (IFNGR1, JAK1 or JAK2) rendered glioblastoma and other solid tumours more resistant to killing by CAR-T cells both in vitro and in vivo(Fig.1). However, loss of this pathway did not render leukaemia or lymphoma cell lines insensitive to CAR-T cells(Fig.1). Using transcriptional profiling, they determined that glioblastoma cells lacking IFNγR1 had lower upregulation of cell-adhesion pathways after exposure to CAR-T cells. In glioblastoma tumours, IFNγR signalling was required for sufficient adhesion of CAR-T cells to mediate productive cytotoxicity. The work demonstrates that liquid and solid tumours differ in their interactions with CAR-T cells and suggests that enhancing binding interactions between T cells and tumour cells may yield improved responses in solid tumours.
2. CAR-T cells expressing a bacterial virulence factor trigger potent bystander antitumour responses in solid cancers
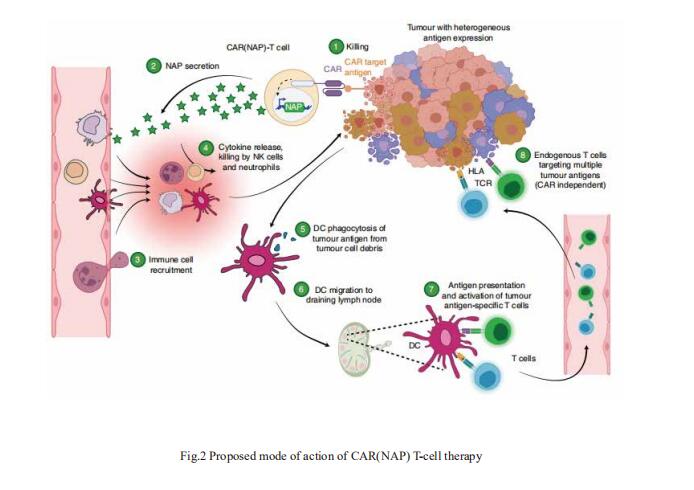
In solid tumours, their potency is hampered by local immunosuppression and by the heterogeneous expression of the antigen that the CAR targets. Magnus Essand, Department of Immunology, Genetics and Pathology, Science for Life Laboratory, Uppsala University, Sweden, and his team showed that CAR-T cells expressing a pluripotent pro-inflammatory neutrophil-activating protein (NAP) from Helicobacter pylori trigger endogenous bystander T-cell responses against solid cancers[2]. In mice with subcutaneous murine pancreatic ductal adenocarcinomas, neuroblastomas or colon carcinomas, CAR(NAP)-T cells led to slower tumour growth and higher survival rates than conventional mouse CAR-T cells, regardless of target antigen, tumour type and host haplotype. In tumours with heterogeneous antigen expression, NAP secretion induced the formation of an immunologically ‘hot’ microenvironment that supported dendritic cell maturation and bystander responses, as indicated by epitope spreading and infiltration of cytotoxic CD8+ T cells targeting tumour-associated antigens other than the CAR-targeted antigen(Fig.2). CAR-T cells armed with NAP neither increased off-tumour toxicity nor hampered the efficacy of CAR-T cells, and hence may have advantageous translational potential.
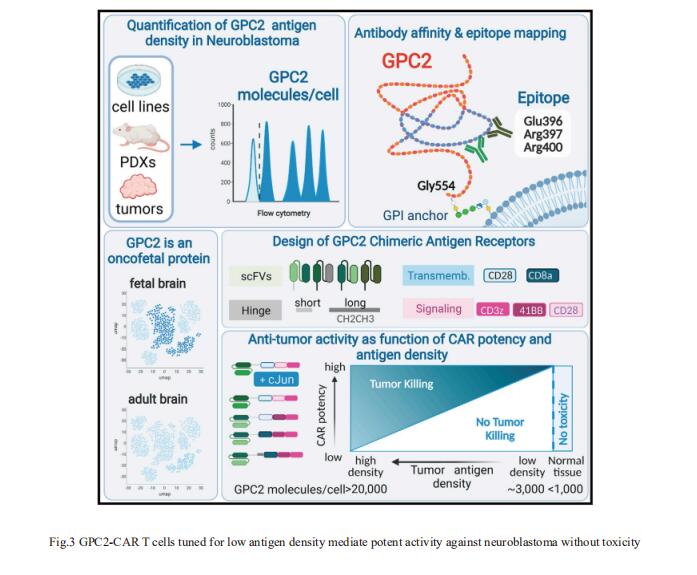
3. GPC2-CAR T cells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity
Pediatric cancers often mimic fetal tissues and express proteins normally silenced postnatally that could serve as immune targets. Crystal L. Mackall, Center for Cancer Cell Therapy, Stanford Cancer Institute, Stanford University School of Medicine, USA, and his team developed T cells expressing chimeric antigen receptors (CARs) targeting glypican-2 (GPC2), a fetal antigen expressed on neuroblastoma (NB) and several other solid tumors[3]. Using flow cytometry to measure GPC2 antigen density on metastatic NBs in pediatric bone marrow (BM) samples, we demonstrate that GPC2 antigen density in clinical specimens is below the threshold required to control tumor growth for traditionally designed GPC2-CAR T cells. Iterative engineering of transmembrane (TM) and co-stimulatory domains plus overexpression of c-Jun lowered the GPC2-CAR antigen density threshold, enabling potent and durable eradication of NBs expressing clinically relevant GPC2 antigen density, without toxicity(Fig.3). These studies highlight the critical interplay between CAR design and antigen density threshold, demonstrate potent efficacy and safety of a lead GPC2-CAR candidate suitable for clinical testing, and credential oncofetal antigens as a promising class of targets for CAR T cell therapy of solid tumors.
References
[1]Larson RC, Kann MC, Bailey SR, et al. CAR T cell killing requires the IFNγR pathway in solid but not liquid tumours[J]. Nature. 2022, 604(7906):563-570. (IF=69.502)
[2]Jin C, Ma J, Ramachandran M, Yu D, Essand M. CAR T cells expressing a bacterial virulence factor trigger potent bystander antitumour responses in solid cancers[J]. Nat Biomed Eng. 2022, 6(7):830-841. (IF=29.235)
[3]Heitzeneder S, Bosse KR, Zhu Z, et al. GPC2-CAR T cells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity[J]. Cancer Cell. 2022, 40(1):53-69.e9. (IF=38.583)
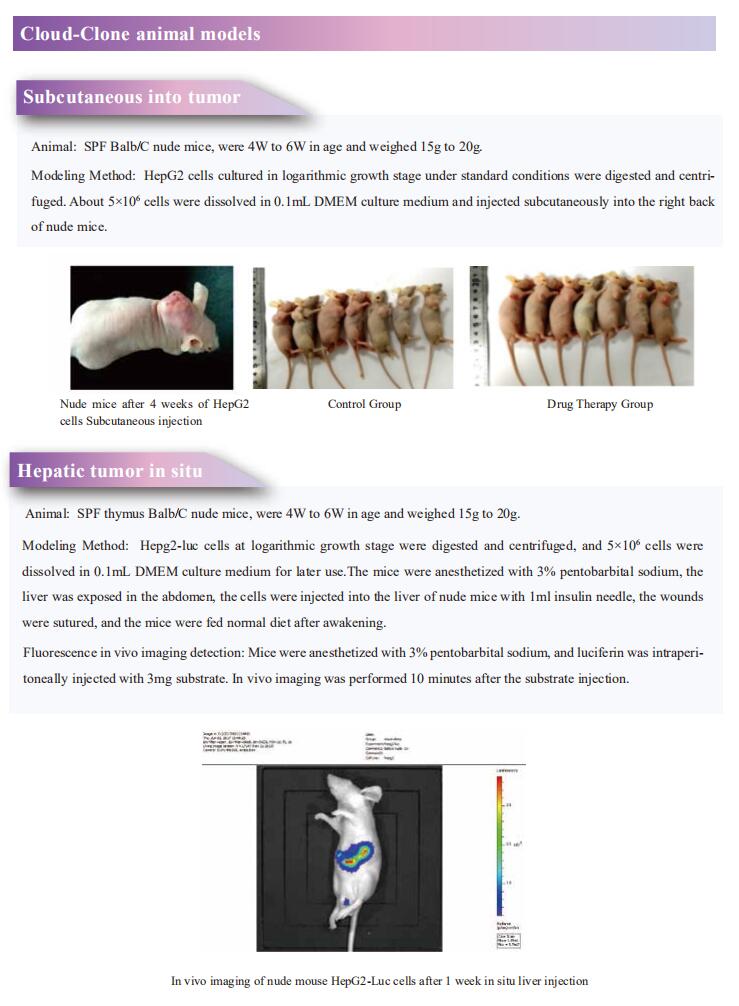
Cloud-Clone can not only provide a variety of experimental tumor animal models, including tumor transplantation animal model, spontaneous tumor animal model, induced tumor animal model, tumor metastasis animal model, covering common tumor research. We also have various cancer detection indicators and the above-mentioned IFNγR signaling pathway and GPC2 related products, which can help the general scientific researchers to carry out cancer related research.