New findings in periodontitis research
Periodontitis is a chronic inflammatory disease with an incidence of over 40% worldwide and has become one of the most common, challenging, and rapidly expanding oral diseases in recent years. It has been considered the main cause of tooth loss and is also connected with several system diseases, such as atherosclerosis and diabetes. Currently, the main strategy for treating periodontitis is to prevent disease progression, including mechanical debridement, antibiotics, anti-inflammatory drugs, and surgical techniques. However, the reconstruction of the periodontal tissue structure is hard to realize since the local infection and inflammation are difficult to eliminate. Recently, a number of papers reported on periodontitis related research, which may provide help for the prevention and treatment of periodontitis.
1. BRD9-mediated chromatin remodeling suppresses osteoclastogenesis through negative feedback mechanism
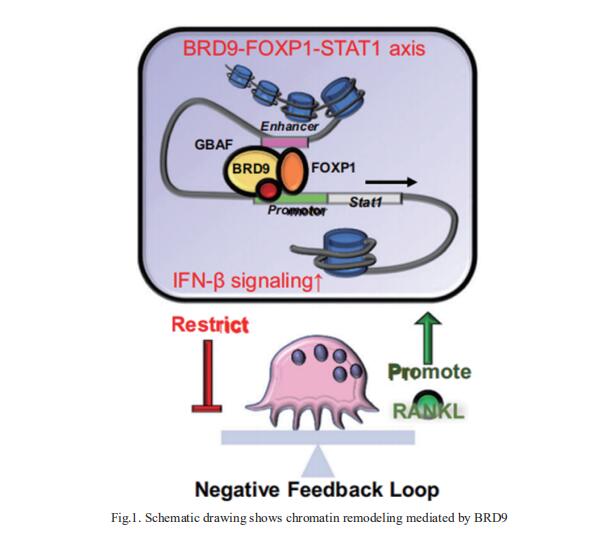
Bromodomain-containing protein 9 (BRD9), a component of non-canonical BAF chromatin remodeling complex. the role of BRD9 in osteoclastogenesis and bone diseases remains unresolved. Xinquan Jiang, Department of Prosthodontics, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, China, and his team showed Brd9 deficiency in myeloid lineage enhances osteoclast lineage commitment and bone resorption through downregulating interferon-beta (IFN-β) signaling with released constraint on osteoclastogenesis[1]. Notably, they showed that BRD9 interacts with transcription factor FOXP1 activating Stat1 transcription and IFN-β signaling thereafter (Fig.1). Leveraging advantages of pharmacological modulation of BRD9 and flexible injectable silk fibroin hydrogel, they designed a local deliver system for effectively mitigating zoledronate related osteonecrosis of the jaw and alleviating acute bone loss in lipopolysaccharide-induced localized aggressive periodontitis. These results demonstrate the function of BRD9 in osteoclastogenesis and its therapeutic potential for bone diseases.
2. Cascade and Ultrafast Artificial Antioxidases Alleviate Inflammation and Bone Resorption in Periodontitis
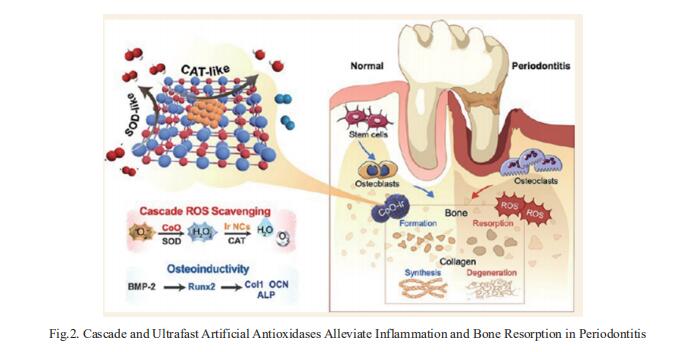
Periodontitis is an oxidative stress-related disease caused by excessive reactive oxygen species (ROS) production. Developing ROS-scavenging materials to regulate the periodontium microenvironments is essential for treating periodontitis. Xianglong Han, State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, China, and his team reported on creating cobalt oxide-supported Ir (CoO−Ir) as a cascade and ultrafast artificial antioxidase to alleviate local tissue inflammation and bone resorption in periodontitis[2]. It is demonstrated that the Ir nanoclusters are uniformly supported on the CoO lattice, and there is stable chemical coupling and strong charge transfer from Co to Ir sites. Benefiting from its structural advantages, CoO−Ir presents cascade and ultrafast superoxide dismutase-catalase-like catalytic activities. Notably, it displays distinctly increased Vmax and turnover number when eliminating H2O2, which surpasses most of the by-far-reported artificial enzymes. Consequently, the CoO−Ir not only provides efficient cellular protection from ROS attack but also promotes osteogenetic differentiation in vitro. Furthermore, CoO−Ir can efficiently combat periodontitis by inhibiting inflammation-induced tissue destruction and promoting osteogenic regeneration (Fig.2). This work will offer inspiration for designing cascade and ultrafast artificial antioxidases for treating abundant oxidative stress-related diseases, such as periodontitis, arthritis, enteritis, and nerve injury.
3. Melatonin Engineering M2 Macrophage-Derived Exosomes for Periodontitis Therapy
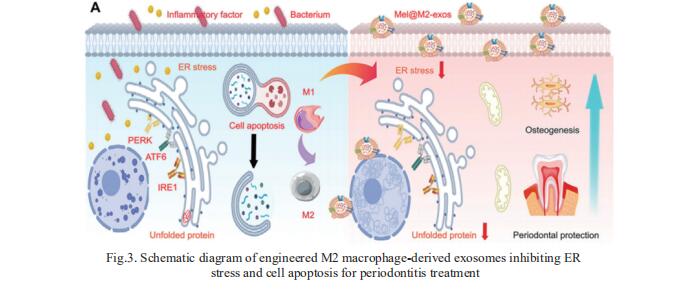
As an essential component of the host immunity, macrophages are highly plastic and play a crucial role in inflammatory response. As M2 macrophage-derived exosomes (M2-exos) can actively target inflammatory sites and modulate immune microenvironments, M2-exos can effectively treat periodontitis. Lixia Mao, Department of Oral and Cranio-Maxillofacial Surgery, Shanghai Ninth People's Hospital, China, and his team fabricated engineered M2-exos loading with melatonin (Mel@M2-exos) for treating periodontitis[3]. As a result, M2-exos drive an appropriate and timely macrophage reprogramming from M1 to M2 type, which resolves chronic inflammation and accelerated periodontal healing. Melatonin released from Mel@M2-exos rescues the osteogenic and cementogenic differentiation capacity in inflammatory human periodontal ligament cells (hPDLCs) by reducing excessive ER stress and unfolded protein response (Fig.3). Injectable gelatin methacryloyl (GelMA) hydrogels with sustained-release Mel@M2-exos accelerate periodontal bone regeneration in rats with ligation-induced periodontitis. Taken together, melatonin engineering M2 macrophage-derived exosomes are promising candidates for inflammatory periodontal tissue regeneration.
4. Neutrophil extracellular traps and extracellular histones potentiate IL-17 inflammation in periodontitis
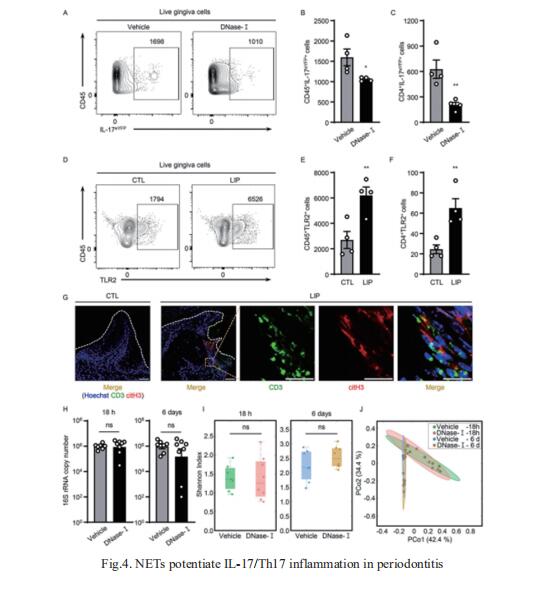
Neutrophil infiltration is a hallmark of periodontitis, a prevalent oral inflammatory condition in which Th17-driven mucosal inflammation leads to destruction of tooth-supporting bone. Niki M. Moutsopoulos, Oral Immunity and Infection Section, National Institute of Dental and Craniofacial Research, National Institutes of Health, USA, and his team documented that neutrophil extracellular traps (NETs) are early triggers of pathogenic inflammation in periodontitis[4]. In an established animal model, they demonstrated that neutrophils infiltrate the gingival oral mucosa at early time points after disease induction and expel NETs to trigger mucosal inflammation and bone destruction in vivo. Investigating mechanisms by which NETs drive inflammatory bone loss, they found that extracellular histones, a major component of NETs, trigger upregulation of IL-17/Th17 responses, and bone destruction. Importantly, human findings corroborate our experimental work (Fig.4). They documented significantly increased levels of NET complexes and extracellular histones bearing classic NET-associated posttranslational modifications, in blood and local lesions of severe periodontitis patients, in the absence of confounding disease. These findings suggest a feed-forward loop in which NETs trigger IL-17 immunity to promote immunopathology in a prevalent human inflammatory disease.
References
[1]Du J, Liu Y, Wu X, et al. BRD9-mediated chromatin remodeling suppresses osteoclastogenesis through negative feedback mechanism. Nat Commun. 2023;14(1):1413. (IF=16.6)
[2]Xie Y, Xiao S, Huang L, et al. Cascade and Ultrafast Artificial Antioxidases Alleviate Inflammation and Bone Resorption in Periodontitis. ACS Nano. 2023;10.1021/acsnano.3c04328. (IF=17.1)
[3]Cui Y, Hong S, Xia Y, et al. Melatonin Engineering M2 Macrophage-Derived Exosomes Mediate Endoplasmic Reticulum Stress and Immune Reprogramming for Periodontitis Therapy. Adv Sci (Weinh). 2023;e2302029. (IF=15.1)
[4]Kim TS, Silva LM, Theofilou VI, et al. Neutrophil extracellular traps and extracellular histones potentiate IL-17 inflammation in periodontitis. J Exp Med. 2023;220(9):e20221751. (IF=15.3)
Cloud-Clone can not only provide animal models of periodontitis, but also has various infection and immune-related protein index detection products, as well as the above-mentioned protein related products such as BRD9, FOXP1, Stat1, IFN-β, melatonin, IL-17, which can help researchers to conduct periodontitis related research.

