New findings in intestinal stem cell research
The intestinal epithelium is part of the mucosa layer. The epithelium contains several cell types including mature secretory cells, mature absorptive cells, progenitors/transit-amplifying cells, and stem cells. Intestinal stem cells (ISCs) are responsible for ongoing epithelial regeneration throughout life. The stem cell-to-daughter cell transition in the intestinal epithelium is believed to be a highly dynamic and plastic process. Stem cell maintenance and the fine-tuning of differentiation into all different lineages are controlled by the surrounding microenvironment, including the cellular and extracellular matrix niche. On a molecular level, multiple signalling pathways together orchestrate cell-cell communication to achieve tissue homeostasis of the gut, and dysregulation of these cascades is commonly associated with intestinal diseases. To unravel the mysteries of intestinal disease mechanisms and stem cell regeneration, insights into ISCs and ISCs niche components are of high importance.
1. Gut microbiota drives macrophage-dependent self-renewal of intestinal stem cells via niche enteric serotonergic neurons
Lgr5+ intestinal stem cells (ISCs) reside within specialized niches at the crypt base and harbor self-renewal and differentiation capacities. Zusen Fan, CAS Key Laboratory of Infection and Immunity, CAS Center for Excellence in Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, China, and his team showed that ISCs are regulated by microbiota and niche enteric serotonergic neurons[1]. The gut microbiota metabolite valeric acid promotes Tph2 expression in enteric serotonergic neurons via blocking the recruitment of the NuRD complex onto Tph2 promoter. 5-hydroxytryptamine (5-HT) in turn activates PGE2 production in a PGE2+ macrophage subset through its receptors HTR2A/3A; and PGE2 via binding its receptors EP1/EP4, promotes Wnt/β-catenin signaling in ISCs to promote their self-renewal (Fig.1). These findings illustrate a complex crosstalk among microbiota, intestinal nerve cells, intestinal immune cells and ISCs, revealing a new layer of ISC regulation by niche cells and microbiota.
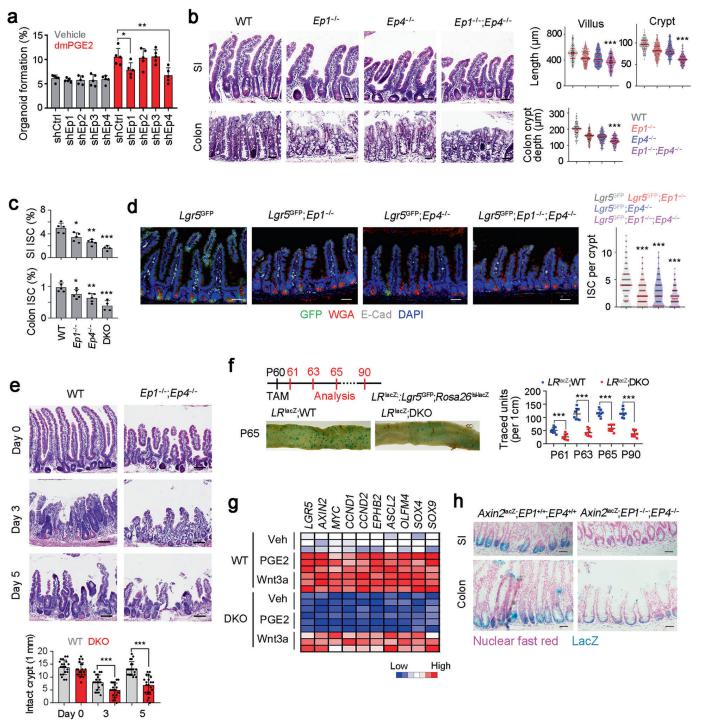
Fig.1 PGE2 drives Wnt signaling activation to maintain ISC stemness
2. Retrograde movements determine effective stem cell numbers in the intestine
The morphology and functionality of the epithelial lining differ along the intestinal tract, but tissue renewal at all sites is driven by stem cells at the base of crypts. Jacco van Rheenen, Department of Molecular Pathology, The Netherlands Cancer Institute, The Netherlands, and his team showed that despite similarities in the number and distribution of proliferative cells with an Lgr5 signature in mice, small intestinal crypts contain twice as many effective stem cells as large intestinal crypts[2]. They found that, although passively displaced by a conveyor-belt-like upward movement, small intestinal cells positioned away from the crypt base can function as long-term effective stem cells owing to Wnt-dependent retrograde cellular movement (Fig.2). By contrast, the near absence of retrograde movement in the large intestine restricts cell repositioning, leading to a reduction in effective stem cell number. Moreover, after suppression of the retrograde movement in the small intestine, the number of effective stem cells is reduced, and the rate of monoclonal conversion of crypts is accelerated. Together, these results showed that the number of effective stem cells is determined by active retrograde movement, revealing a new channel of stem cell regulation that can be experimentally and pharmacologically manipulated.
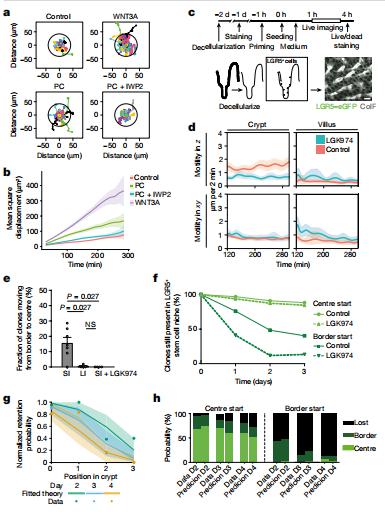
Fig.2 Wnt promotes LGR5+ cell migration
3. Screening for modulators of the cellular composition of gut epithelia via organoid models of intestinal stem cell differentiation
The cellular composition of barrier epithelia is essential to organismal homoeostasis. Yet, methods for the identification of biological targets regulating epithelial composition and function, and of small molecules modulating them, are lacking. Alex K. Shalek, Harvard-MIT Program in Health Sciences and Technology, USA, and his team showed that druggable biological targets and small-molecule regulators of intestinal stem cell differentiation can be identified via multiplexed phenotypic screening using thousands of miniaturized organoid models of intestinal stem cell differentiation into Paneth cells, and validated via longitudinal single-cell RNA-sequencing[3]. They found that inhibitors of the nuclear exporter Exportin 1 (XPO1) modulate the fate of intestinal stem cells, independently of known differentiation cues, significantly increasing the abundance of Paneth cells in the organoids and in wild-type mice (Fig.3). Physiological organoid models of the differentiation of intestinal stem cells could find broader utility for the screening of biological targets and small molecules that can modulate the composition and function of other barrier epithelia.
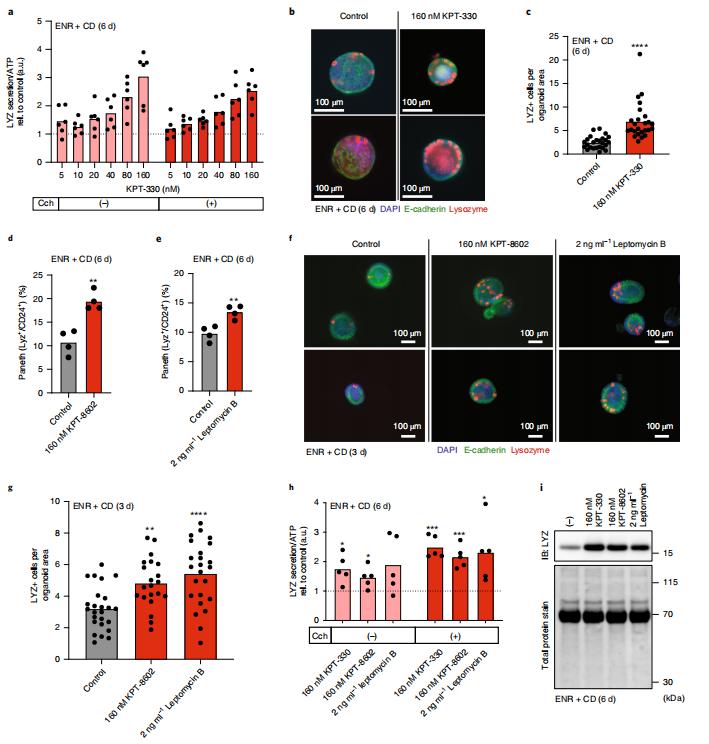
Fig.3 Small-molecule inhibition of XPO1 enhances Paneth cell differentiation
4. Lymphatics act as a signaling hub to regulate intestinal stem cell activity
Epithelial stem cells of small and large intestines (ISCs) respond to their local microenvironments (niches) to fulfill a continuous demand for tissue turnover. Elaine Fuchs, Robin Chemers Neustein Laboratory of Mammalian Cell Biology and Development, The Rockefeller University, USA, and his team found that lymphatics at the base of intestinal crypts function as a signaling hub for the crypt in general and ISCs in particular (Fig.4)[4]. Organoid cocultures studies showed that lymphatic-derived ligands act directly on ISCs and maintain their self-renewal ability and fitness, although concurrently suppressing differentiation. They identified WNT-signaling factors (WNT2, R-SPONDIN-3) and a hitherto unappreciated extracellular matrix protein, REELIN, as crypt lymphatic signals that directly govern the regenerative potential of ISCs. With the diversity of roles that lymphatics can play, their association with ISCs is likely to have important consequences in disease states.
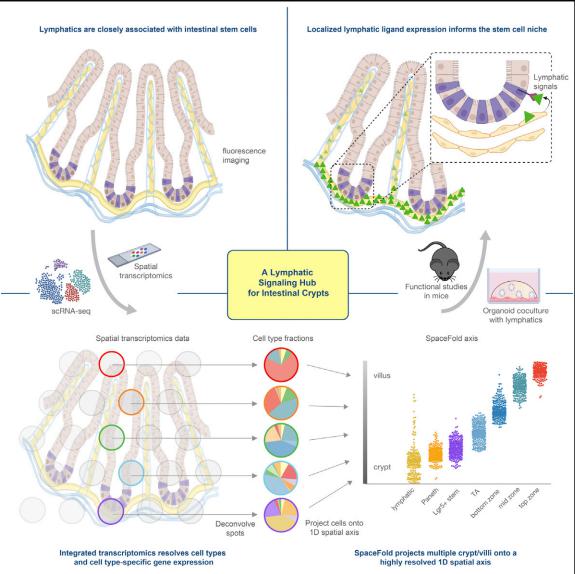
Fig.4 Lymphatics act as a signaling hub to regulate intestinal stem cell activity
References
[1]Zhu P, Lu T, Wu J, et al. Gut microbiota drives macrophage-dependent self-renewal of intestinal stem cells via niche enteric serotonergic neurons. Cell Res. 2022;32(6):555-569. (IF=46.297)
[2]Azkanaz M, Corominas-Murtra B, Ellenbroek SIJ, et al. Retrograde movements determine effective stem cell numbers in the intestine. Nature. 2022;607(7919):548-554. (IF=69.504)
[3]Mead BE, Hattori K, Levy L, et al. Screening for modulators of the cellular composition of gut epithelia via organoid models of intestinal stem cell differentiation. Nat Biomed Eng. 2022;6(4):476-494. (IF=29.234)
[4]Niec RE, Chu T, Schernthanner M, et al. Lymphatics act as a signaling hub to regulate intestinal stem cell activity. Cell Stem Cell. 2022;29(7):1067-1082.e18. (IF=25.269)
Cloud-Clone not only provides animal models of various digestive system diseases, including gastric ulcer, colitis, irritable bowel syndrome, gastritis, duodenal ulcer and other common digestive disease animal models. We also have various digestive system diseases detection indicators and the above HTR2A/3A, PGE2, Wnt/β-catenin, XPO1 and other related products, which can help the vast number of researchers to carry out digestive system diseases related research.