New findings in bone regeneration
Bone tissue is a hard connective tissue of the human body and is constantly reshaped throughout the life of an individual. Bone tissue exerts both supporting and protective effects and can protect fragile organs within the body. When bone defect areas are small, most bones can undergo self-healing without any treatment. However, when bone tissue suffers damage beyond its ability to repair itself, bone damage can occur. Severe bone defects can be caused by tumor resection, severe trauma, infection, congenital malformation, osteogenesis imperfecta, rheumatoid arthritis, and osteoporosis, thus requiring clinical intervention. However, despite the high incidence of bone injury, treatment selection remains controversial. Recently, multiple studies have reported on bone repair and regeneration, which may help develop new clinical intervention methods for bone repair.
1. A Mechanically Reinforced Super Bone Glue Makes a Leap in Hard Tissue Strong Adhesion and Augmented Bone Regeneration
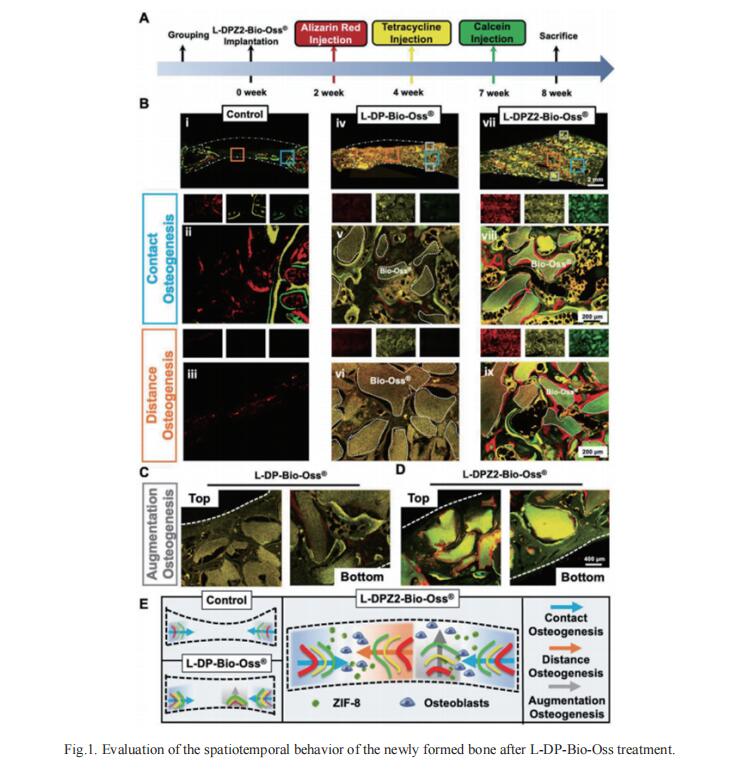
Existing bone tissue engineering strategies aim to achieve minimize surgical trauma, stabilize the injured area, and establish a dynamic osteogenic microenvironment. Inspired by the excellent adhesive properties of mussels, Tao Chen, Stomatological Hospital of Chongqing Medical University, China, and his team used a super osteogenic glue (L-DPZ) that integrates poly(vinyl alcohol), L-dopa amino acid, and zeolitic imidazolate framework-8 characterized by catechol–metal coordination, successfully adhere to hard tissue with a maximum adhesive strength of 10 MPa, which is much higher than those of commercial and previously reported bone glues[1]. The stable hard tissue adhesion also enables it to adhere strongly to luxated or broken teeth, Bio-Oss (a typical bone graft material), and splice fragments from comminuted fractures of the rabbit femur. Then, it is testified that the L-DPZ hydrogels exhibit satisfactory biocompatibility, stable degradability, and osteogenic ability in vitro. Moreover, the ability to anchor Bio-Oss and sustained osteogenesis of L-DPZ result in satisfactory healing in calvarial bone defect models in rabbits, as observed by increased bone thickness and the ingrowth of new bone tissue (Fig.1). These results are expected to demonstrate solutions to clinical dilemmas such as comminuted bone fracture fixation, bone defect reconstruction, and teeth dislocation replantation.
2. A MgFe-LDH Nanosheet-Incorporated Smart ThermoResponsive Hydrogel with Controllable Growth Factor Releasing Capability for Bone Regeneration
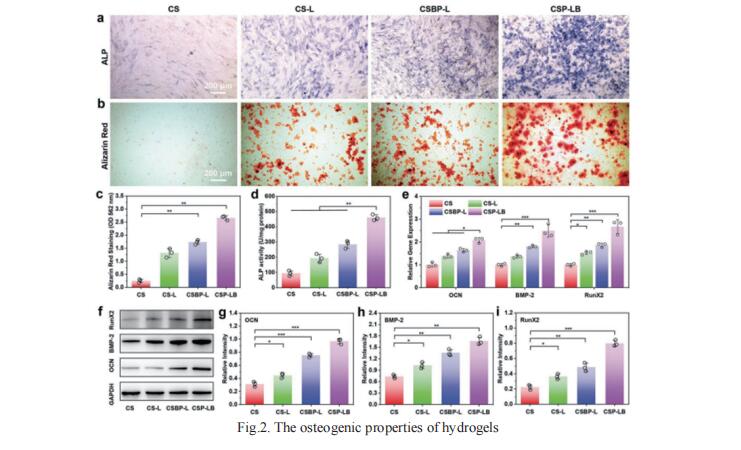
Although growth factor (GF)-loaded hydrogels have been explored as promising materials in repairing bone defects, it still remains challenging to construct smart hydrogels with excellent gelation/mechanical properties as well as controllable GF releasing capability. Xisheng Weng, Department of Orthopedic Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, China, and his team constructed a smart injectable thermo-responsive hydrogel (denoted as CSP-LB) by incorporating bone morphogenetic protein 2 (BMP-2) functionalized MgFe layered double hydroxide (LDH) nanosheets into chitosan/silk fibroin (CS) hydrogel loaded with platelet-derived growth factor BB (PDGF-BB), which can achieve a burst release of PDGF-BB and a sustained release of BMP-2, for highly efficient bone regeneration is reported[2]. The incorporation of MgFe-LDH in CS hydrogel not only shortens the gelation time and decreases sol–gel transition temperature, but also enhances the mechanical property of the hydrogel. Because of the sequential release of dual-GFs and sustained release of bioactive Mg2+/Fe3+ ions, the in vitro experiments prove that the CSP-LB hydrogel exhibits excellent angiogenic and osteogenic properties compared with the CS hydrogel (Fig.2). In vivo experiments further prove that the CSP-LB hydrogel can significantly enhance bone regeneration with higher bone volume and mineral density than that of the CS hydrogel. This smart thermo-sensitive CSP-LB hydrogel possesses excellent gelation capability and angiogenic and osteogenic properties, thus providing a promising minimally invasive solution for bone defect treatment.
3. Engineered Sensory Nerve Guides Self-Adaptive Bone Healing via NGF-TrkA Signaling Pathway
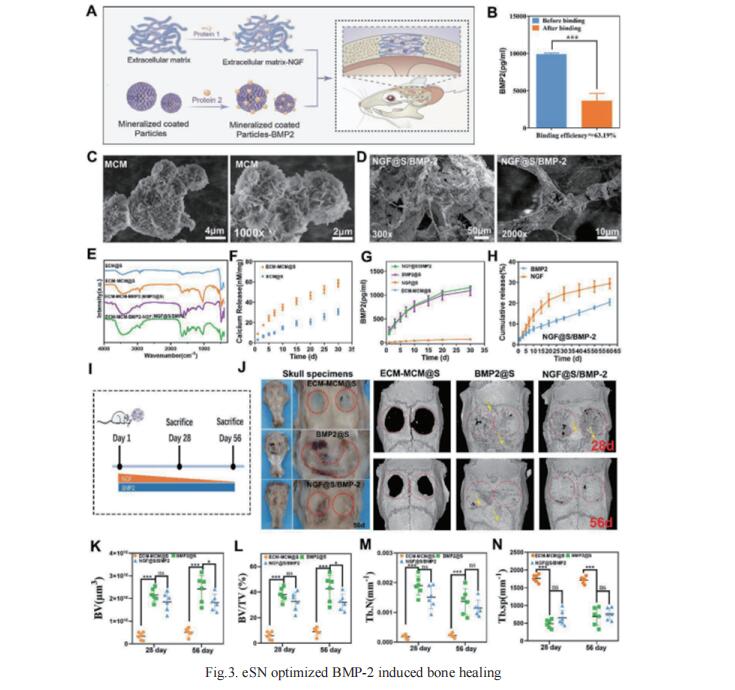
The upstream role of sensory innervation during bone homeostasis is widely underestimated in bone repairing strategies. Xiaohua Yu, Department of Orthopedic Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, China, and his team proposed a neuromodulation approach to orchestrate bone defect healing by constructing engineered sensory nerves (eSN) in situ to leverage the adaptation feature of SN during tissue formation[3]. NGF liberated from ECM-constructed eSN effectively promotes sensory neuron differentiation and enhances CGRP secretion, which lead to improved RAOECs mobility and osteogenic differentiation of BMSC. In turn, such eSN effectively drives ossification in vivo via NGF-TrkA signaling pathway, which substantially accelerates critical size bone defect healing. More importantly, eSN also adaptively suppresses excessive bone formation and promotes bone remodeling by activating osteoclasts via CGRP-dependent mechanism when combined with BMP-2 delivery, which ingeniously alleviates side effects of BMP-2 (Fig.3). In sum, this eSN approach offers a valuable avenue to harness the adaptive role of neural system to optimize bone homeostasis under various clinical scenario.
4. Stem Cell Membrane-Coated Microribbon Scaffolds Induce Regenerative Innate and Adaptive Immune Responses
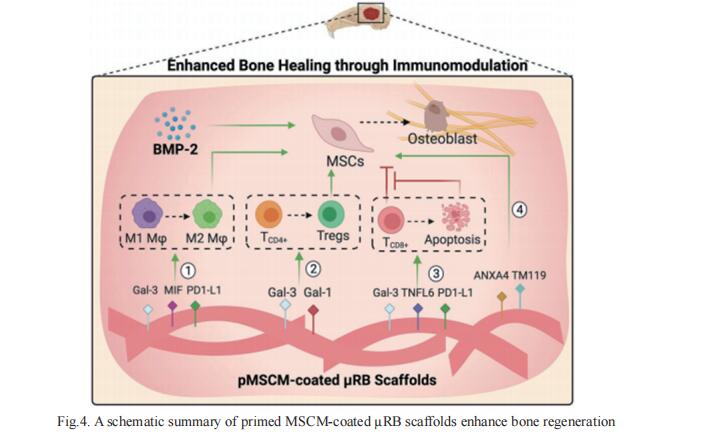
Naturally-derived cell membranes have shown great promise in functionalizing nanoparticles to enhance biointerfacing functions for drug delivery applications. However, its potential for functionalizing macroporous scaffolds to enhance tissue regeneration in vivo remains unexplored. Fan Yang, Department of Orthopaedic Surgery, Stanford University School of Medicine, USA, and his team reported mensenchymal stem cell membrane (MSCM)-coated microribbon (µRB) scaffolds for treating critical size cranial bone defects via targeting immunomodulation[4]. It is demonstrated that MSCM coating promotes macrophage (Mϕ) polarization toward regenerative phenotype, induces CD8+ T cell apoptosis, and enhances regulatory T cell differentiation in vitro and in vivo. When combined with a low dosage of BMP-2, MSCM coating further accelerates bone regeneration and suppresses inflammation (Fig.4). These results establish cell membrane-coated microribbon scaffolds as a promising strategy for treating critical size bone defects via immunomodulation.
References
[1]Hu S, Wang S, He Q, et al. A Mechanically Reinforced Super Bone Glue Makes a Leap in Hard Tissue Strong Adhesion and Augmented Bone Regeneration. Adv Sci (Weinh). 2023;10(11):e2206450. (IF-17.521)
[2]Lv Z, Hu T, Bian Y, et al. A MgFe-LDH Nanosheet-Incorporated Smart Thermo-Responsive Hydrogel with Controllable Growth Factor Releasing Capability for Bone Regeneration. Adv Mater. 2023;35(5):e2206545. (IF=32.086)
[3]Zhang Z, Wang F, Huang X, et al. Engineered Sensory Nerve Guides Self-Adaptive Bone Healing via NGF-TrkA Signaling Pathway. Adv Sci (Weinh). 2023;10(10):e2206155. (IF-17.521)
[4]Su N, Villicana C, Barati D, Freeman P, Luo Y, Yang F. Stem Cell Membrane-Coated Microribbon Scaffolds Induce Regenerative Innate and Adaptive Immune Responses in a Critical-Size Cranial Bone Defect Model. Adv Mater. 2023;35(10):e2208781. (IF=32.086)
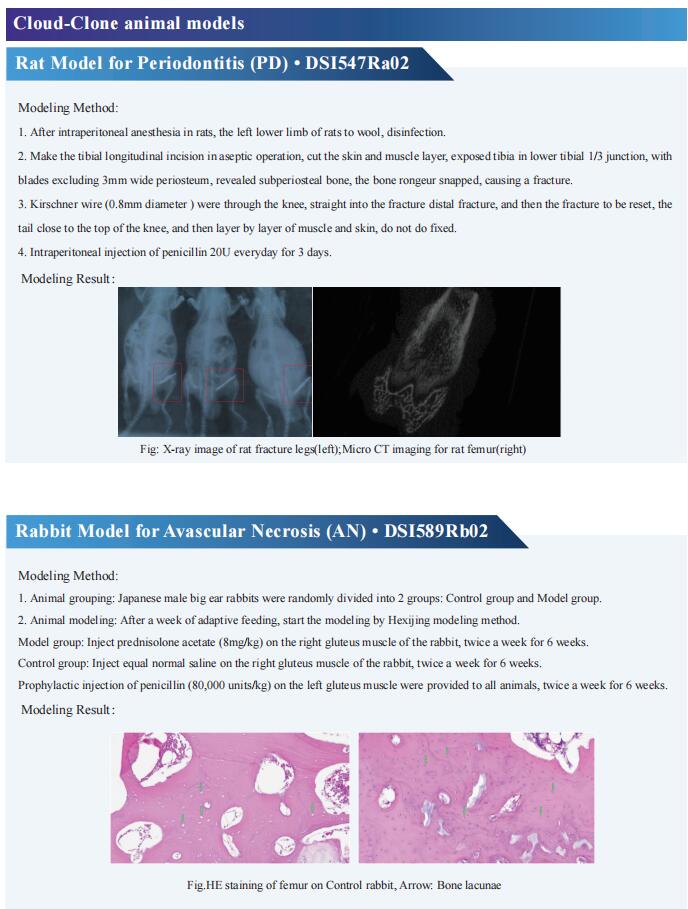
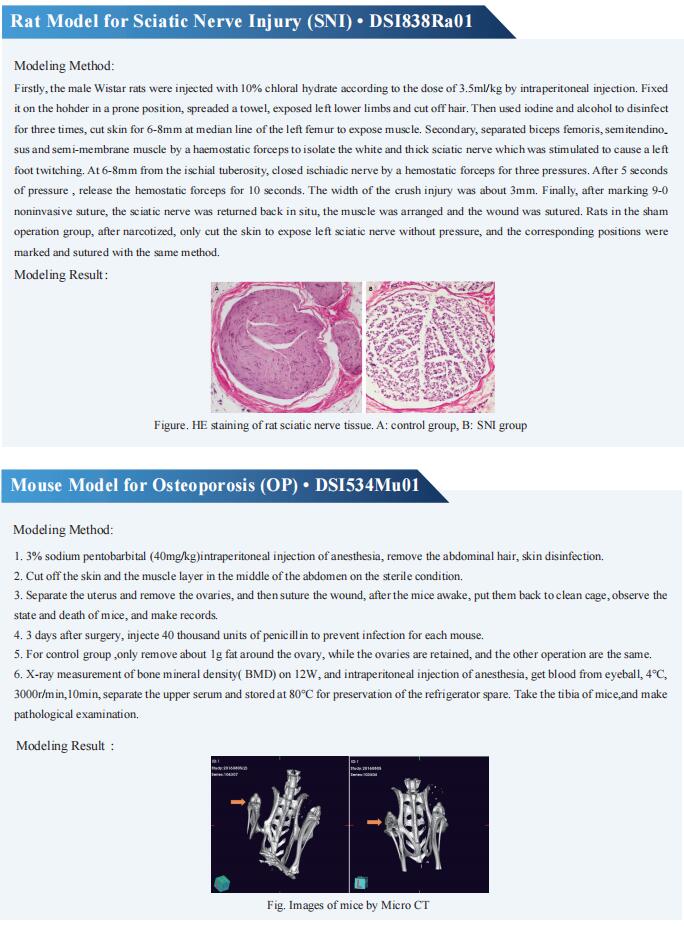
Cloud-Clone can not only provide a variety of animal models of bone diseases, including femoral defect, skull defect, osteoporosis, fracture, articular cartilage injury, rheumatoid arthritis, osteoarthritis and other common bone diseases. We also have various bone metabolism detection indicators and the above-mentioned BMP-2, PDGF-BB, CGRP, NGF and other related products, which can help the majority of scientific researchers to conduct research on the treatment of bone diseases.