New discovery of the pathological mechanism of alcoholic liver disease
Alcohol-associated liver disease (ALD) is a leading cause of liver injury resulting from chronic and abusive alcohol consumption. The spectrum of ALD ranges from alcoholic steatosis, to hepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). A wide variety of host and environmental factors and comorbidities have been shown to modify the development and progression of ALD, including age, sex, genetic factors, drinking pattern, obesity, and chronic viral hepatitis. Despite major advances made in identifying potential pathways and therapeutic targets, there is no effective therapy besides abstinence. Uncovering novel molecular mechanisms of ALD pathogenesis that can lead to new treatments are urgently needed.
1. Aging exaggerates alcohol-induced liver injury by inhibiting neutrophilic sirtuin 1-C/EBPα-miRNA-223 axis
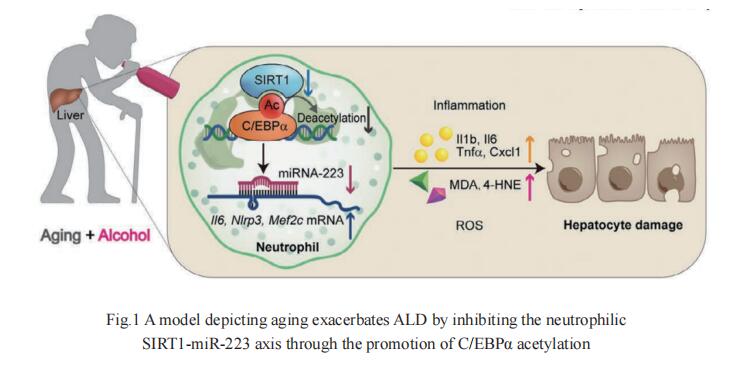
Aging exacerbates liver neutrophil infiltration and ALD in mice and humans, but the underlying mechanisms remain obscure. Hua Wang, Department of Oncology, the First Affiliated Hospital of Anhui Medical University, and his team aimed to examine the effect of aging and alcohol consumption on neutrophilic Sirtuin 1 (SIRT1) and microRNA-223 (miR-223), and their contribution to ALD pathogeneses[1]. Young and aged myeloid-specific Sirt1 knockout mice were subjected to chronic-plus-binge ethanol feeding. Blood samples from healthy controls and patients with chronic alcohol drinking who presented with acute intoxication were analyzed. Neutrophilic Sirt1 and miR-223 expression were down-regulated in aged mice compared with young mice. Deletion of the Sirt1 gene in myeloid cells including neutrophils exacerbated chronicplus-binge ethanol-induced liver injury and inflammation and down-regulated neutrophilic miR-223 expression. Immunoprecipitation experiments revealed that SIRT1 promoted C/EBPα deacetylation by directly interacting with C/EBPα, a key transcription factor that controls miR-223 biogenesis, and subsequently elevated miR-223 expression in neutrophils (Fig.1). Chronic alcohol users with acute intoxication had a reduction in neutrophilic SIRT1 expression in young and middle-aged patients, with a greater reduction in the latter group. The neutrophilic SIRT1 expression correlated with neutrophilic miR-223 and serum alanine transaminase levels in those patients. Aging increases the susceptibility of alcohol-induced liver injury in mice and humans through the down-regulation of the neutrophilic SIRT1-C/EBPα-miR-223 axis, which could be a therapeutic target for the prevention and/or treatment of ALD.
2. Depletion of mitochondrial methionine adenosyltransferase α1 triggers mitochondrial dysfunction in ALD
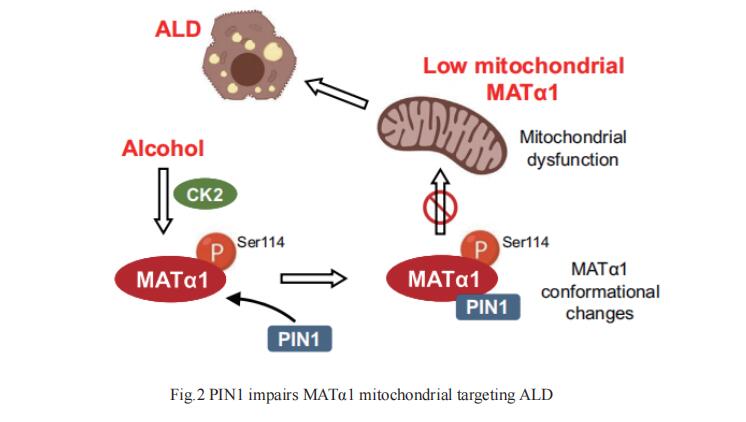
MATα1 catalyzes the synthesis of S-adenosylmethionine, the principal biological methyl donor. Lower MATα1 activity and mitochondrial dysfunction occur in ALD. Shelly C. Lu, Cedars-Sinai Medical Center, Los Angeles, USA, and his team showed that mitochondrial MATα1 is selectively depleted in alcohol-associated liver disease through a mechanism that involves the isomerase PIN1 and the kinase CK2[2]. Alcohol activates CK2, which phosphorylates MATα1 at Ser114 facilitating interaction with PIN1, thereby inhibiting its mitochondrial localization (Fig.2). Blocking PIN1-MATα1 interaction increased mitochondrial MATα1 levels and protected against alcohol-induced mitochondrial dysfunction and fat accumulation. Normally, MATα1 interacts with mitochondrial proteins involved in TCA cycle, oxidative phosphorylation, and fatty acid β-oxidation. Preserving mitochondrial MATα1 content correlates with higher methylation and expression of mitochondrial proteins. Collectively, the study revealed that PIN1 negatively regulates MATα1 mitochondrial localization, and that alcohol enhances PIN1-MATα1 interaction resulting in selective depletion of mitochondrial MATα1, which may play an important role in ALD pathogenesis.
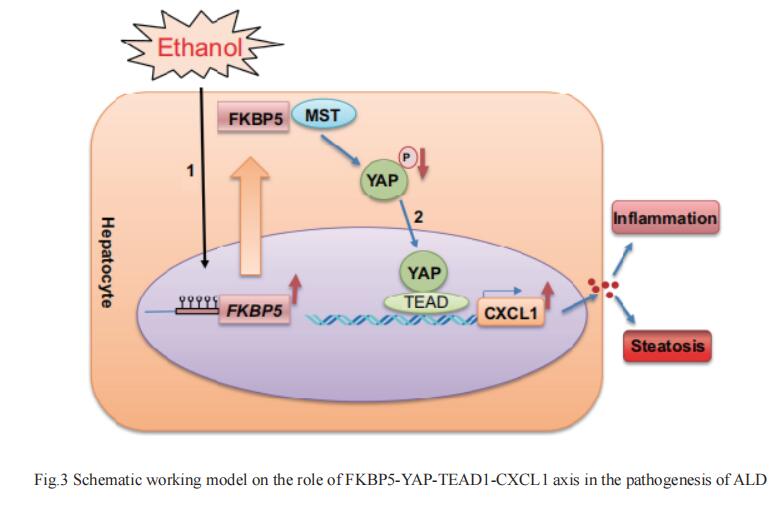
3. Stress-responsive gene FKBP5 mediates alcohol-induced liver injury through the hippo pathway and CXCL1 signaling
Chronic alcohol drinking is a major risk factor for alcohol-associated liver disease (ALD). FK506-binding protein 51 (FKBP5), a co-chaperone protein, is involved in many key regulatory pathways. It is known to be involved in stress-related disorders but there are no reports regarding its role in ALD. Suthat Liangpunsakul, Indiana University School of Medicine, USA, and his team aimed to examine the molecular mechanism of FKBP5 in ALD[3]. They found a significant increase in hepatic FKBP5 transcripts and protein expression in patients with ALD and mice fed with chronic-plus-single binge ethanol. Loss of Fkbp5 in mice protected against alcohol-induced hepatic steatosis and inflammation. Transcriptomic analysis revealed a significant reduction of Tead1 and Cxcl1 mRNA in ethanol-fed Fkbp5-/- mice. Ethanol-induced Fkbp5 expression was secondary to downregulation of methylation level at its 5′ UTR promoter region. The increase in Fkbp5 expression led to induction in transcription factor Tead1 through Hippo signaling pathway. Fkbp5 can interact with YAP upstream kinase, MST1, affecting its ability to phosphorylate YAP and the inhibitory effect of hepatic YAP phosphorylation by ethanol leading to YAP nuclear translocation and TEAD1 activation (Fig.3). Activation of TEAD1 led to increased expression of its novel target, CXCL1, a chemokine-mediated neutrophil recruitment, causing hepatic inflammation and neutrophil infiltration in our mouse model. Loss of FKBP5 ameliorates alcohol-induced liver injury through the Hippo pathway and CXCL1 signaling, suggesting its potential role as a target for the treatment of ALD.
References
[1]Ren R, He Y, Ding D, et al. Aging exaggerates acute-on-chronic alcohol-induced liver injury in mice and humans by inhibiting neutrophilic sirtuin 1-C/EBPα-miRNA-223 axis [J]. Hepatology. 2022, 75(3):646-660. (IF=17.425)
[2]Barbier-Torres L, Murray B, Yang JW, et al. Depletion of mitochondrial methionine adenosyltransferase α1 triggers mitochondrial dysfunction in alcohol-associated liver disease [J]. Nat Commun. 2022, 13(1):557. (IF=14.919)
[3]Kusumanchi P, Liang T, Zhang T, et al. Stress-Responsive Gene FK506-Binding Protein 51 Mediates Alcohol-Induced Liver Injury Through the Hippo Pathway and Chemokine (C-X-C Motif) Ligand 1 Signaling [J]. Hepatology. 2021, 74(3):1234-1250. (IF=17.425)
Cloud-Clone not only provides animal models of various liver diseases, including liver fibrosis, liver ischemia, alcoholic liver disease, intrahepatic cholestasis, liver cirrhosis, fatty liver, liver failure, etc., covering common liver diseases. It also has a variety of commonly used detection indicators for liver diseases and the above-mentioned protein-related products such as SIRT1, C/EBPα, MATα1, PIN1, CK2, FKBP5, TEAD1, YAP, CXCL1, etc., which can help scientific researchers to carry out liver disease research.