Effect of Extracellular Ribonucleic Acids on Neurovascularization in Osteoarthritis

Osteoarthritis (OA), the most common form of arthritis, affects more than 7% of the global population. The prevalence of osteoarthritis is high in individuals of advanced age (>65 years). OA is a typical degenerative disease characterized by abnormal neurovascularization. The invasion of nerves and vessels in the osteochondral junction is one of its hallmarks and is the primary reason for aggravated pain. Anti-inflammatory drug therapy and surgery are the major treatment modalities for OA. However, until recently, long-term disease remission has not been achieved, and the uncertain pathophysiological mechanisms of OA limit treatment options. Many cytokines, including semaphorins, netrins, and growth factors such as vascular endothelial growth factor (VEGF) and nerve growth factor, have been found to play important roles in neurovascularization. Although the inhibition of a single cytokine does not completely reverse pain and OA progression, it is practically impossible to inhibit all cytokines involved. These issues have spurred clinical scientists to question whether other cofactors may be involved in the regulation of neurovascularization in OA.
On July 3, 2023, Kai Jiao, Department of Stomatology, Tangdu hospital, The Fourth Military Medical University, China, and his team published a paper titled “Effect of Extracellular Ribonucleic Acids on Neurovascularization in Osteoarthritis” in Advanced Science. The results of the study suggest that exRNAs may be potential targets for regulating nerve and blood vessel ingrowth under physiological and pathological joint conditions.

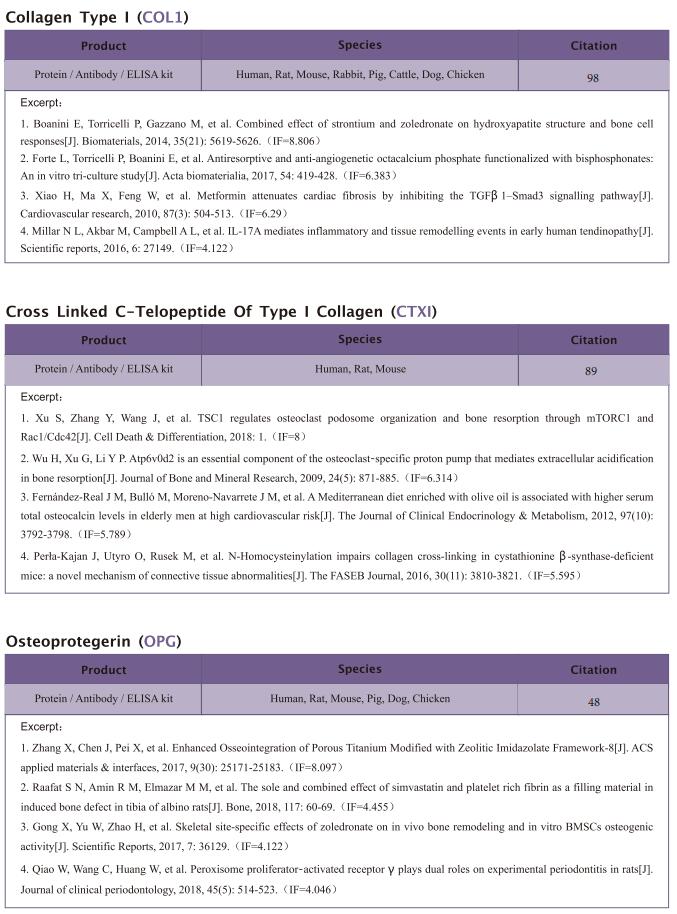
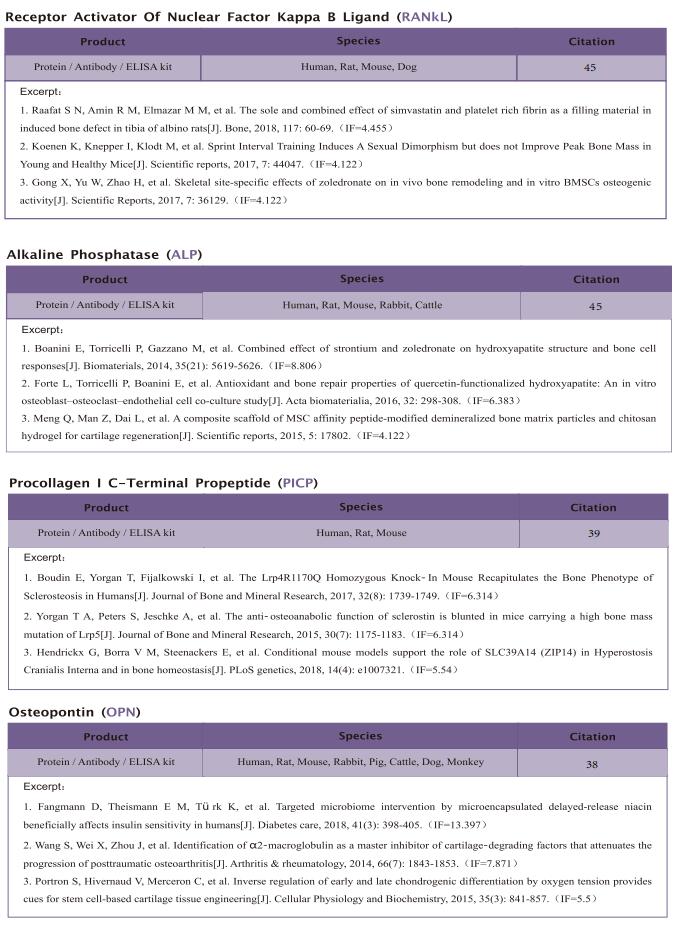
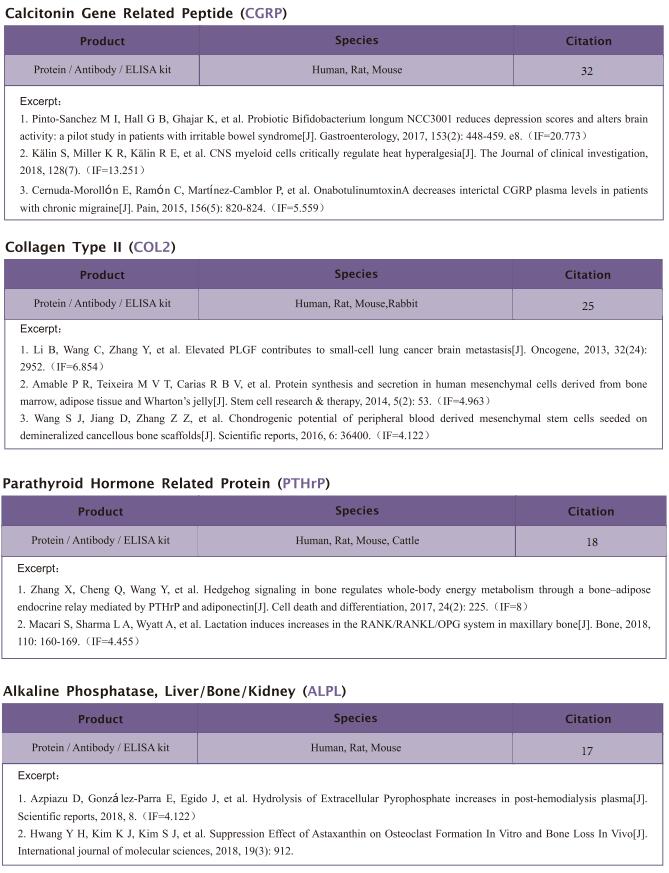
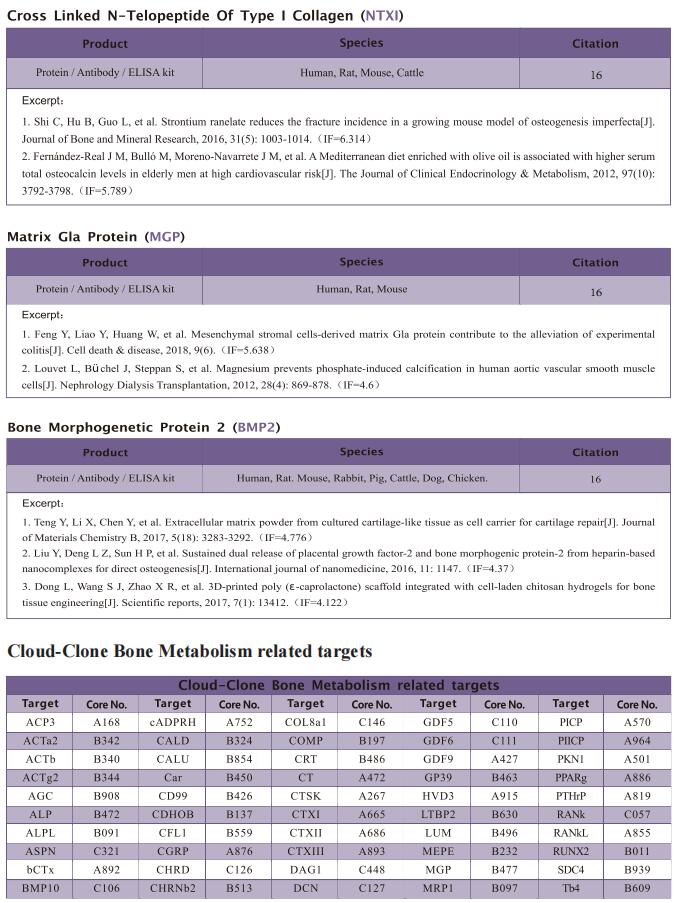
The kit [ELISA Kit for Ribonuclease A (RNase A), SEA297Mu] of Cloud-Clone brand was chosed in this article, we are so proud for supporting the reaserchers.

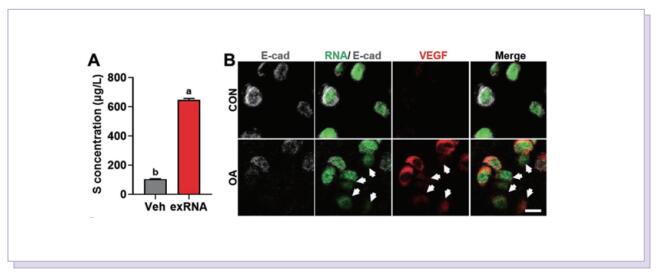
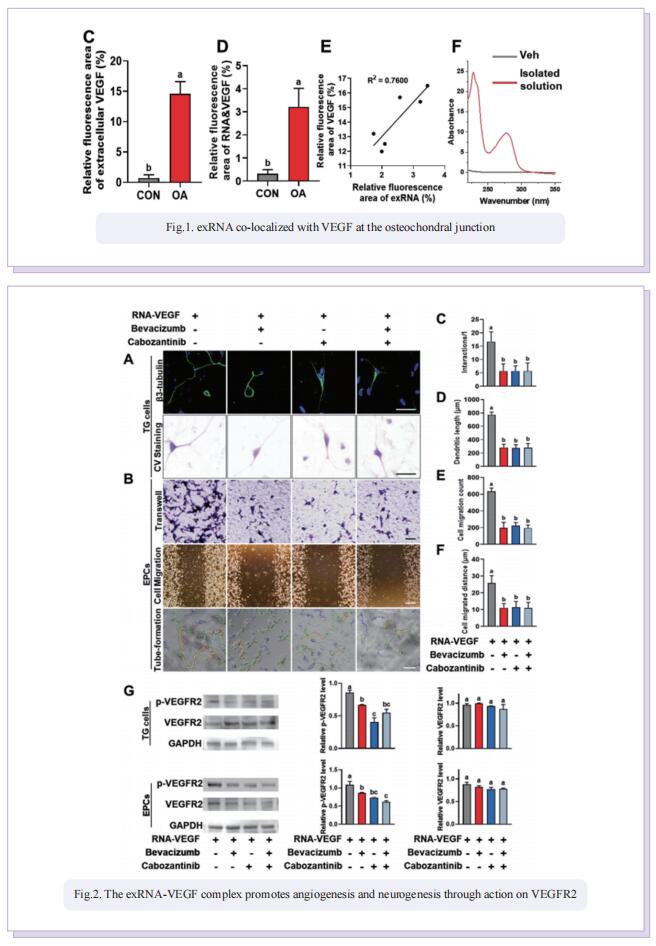
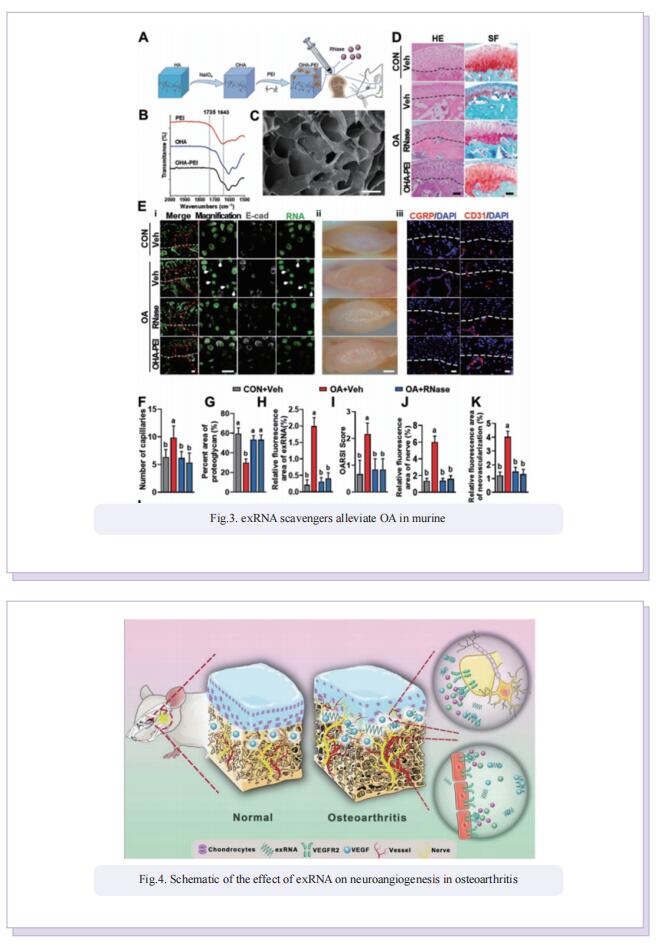
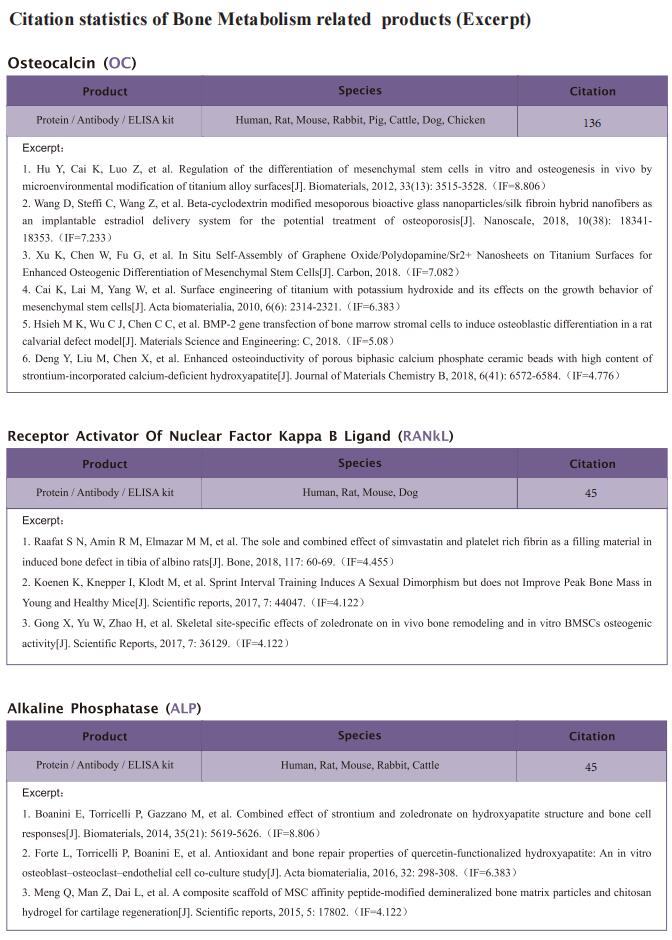
Accordingly, the objective of this study was to test the central hypothesis that extracellular ribonucleic acids (exRNAs) are involved in VEGF function and neurovascular ingrowth during OA progression. A murine osteoarthritic model with augmented neurovascularization at the osteochondral junction was used to examine this under-evaluated facet of degenerative joint dysfunction. Increased extracellular RNA (exRNA) content is identified in neurovascularized osteoarthritic joints. It is found that the amount of exRNA is positively correlated with the extent of neurovascularization and the expression of VEGF. In vitro binding assay and molecular docking demonstrate that synthetic RNAs bind to VEGF via electrostatic interactions. The RNA-VEGF complex promotes the migration and function of endothelial progenitor cells and trigeminal ganglion cells. The use of VEGF and VEGFR2 inhibitors significantly inhibits the amplification of the RNA-VEGF complex. Disruption of the RNA-VEGF complex by RNase and polyethyleneimine reduces its in vitro activities, as well as prevents excessive neurovascularization and osteochondral deterioration in vivo.
In summary, the study showed that exRNAs have the ability to recruit polycationic neurovascular factors, such as VEGF. Recruitment of VEGF amplified its effect on abnormal neurogenesis and angiogenesis in the osteoarthritic condylar joint. This study provides new insights into the mechanisms underlying neurovascularization, and further promotes the clearance of exRNAs via RNase or cationic nanoparticles as a prototype for the design of clinical strategies for treating neurovascularization-related diseases.