Cancer vaccines From theory to practice, ushering in a new era of cancer prevention
When one hears the word “vaccine,” many people think of vaccines against infectious agents, such as viruses and bacteria. The idea of vaccination against cancer has a long history and was initially built on the observation that some tumors spontaneously regress in patients experiencing an acute infection. How a non-specific innate immune response against bacterial products could translate into a specific anti-tumor immune response was explained subsequently by the discovery that dendritic cells (DCs) could acquire immunogenic tumor-derived peptides released during the innate immune response. This led to the hypothesis that use of tumor-derived antigens, if delivered to the immune system in a sufficiently immunogenic context (“vaccine”), would, due to the preferential targeting of cancer cells, enable relatively safe and yet effective treatments for cancer, capable of inducing long-lasting immunity. Based on this foundation, cancer vaccines have been the subject of preclinical and clinical research for a variety of malignancies over the past decades. Recently, a number of articles have reported research related to cancer vaccines, which may help in the treatment of related cancers.
1. An engineered influenza virus to deliver antigens for lung cancer vaccination
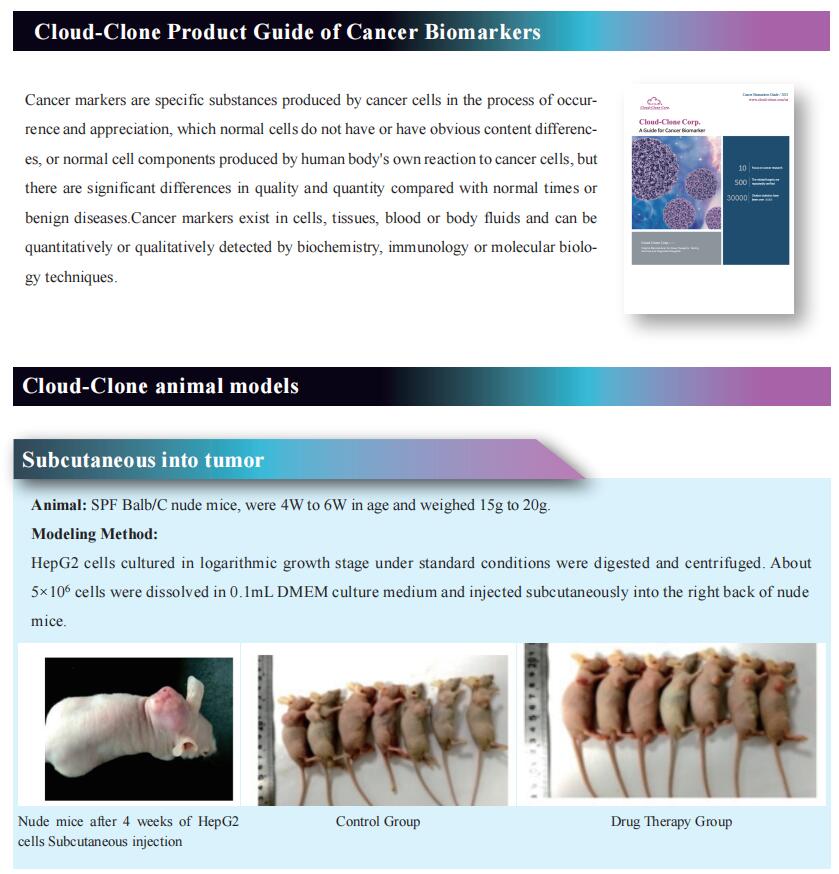
The development of cancer neoantigen vaccines that prime the anti-tumor immune responses has been hindered in part by challenges in delivery of neoantigens to the tumor. Demin Zhou, State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, China, and his team demonstrated a chimeric antigenic peptide influenza virus (CAP-Flu) system for delivery of antigenic peptides bound to infuenza A virus (IAV) to the lung by using the model antigen ovalbumin (OVA) in a melanoma model[1]. They conjugated attenuated IAVs with the innate immunostimulatory agent CpG (Fig.1) and, after intranasal administration to the mouse lung, observed increased immune cell infiltration to the tumor. OVA was then covalently displayed on IAV-CPG using click chemistry. Vaccination with this construct yielded robust antigen uptake by dendritic cells, a specific immune cell response and a significant increase in tumor-infiltrating lymphocytes compared to peptides alone. Lastly, they engineered the IAV to express anti-PD1-L1 nanobodies that further enhanced regression of lung metastases and prolonged mouse survival after rechallenge. Engineered IAVs can be equipped with any tumor neoantigen of interest to generate lung cancer vaccines.
2. Engineered skin bacteria induce antitumor T cell responses against melanoma
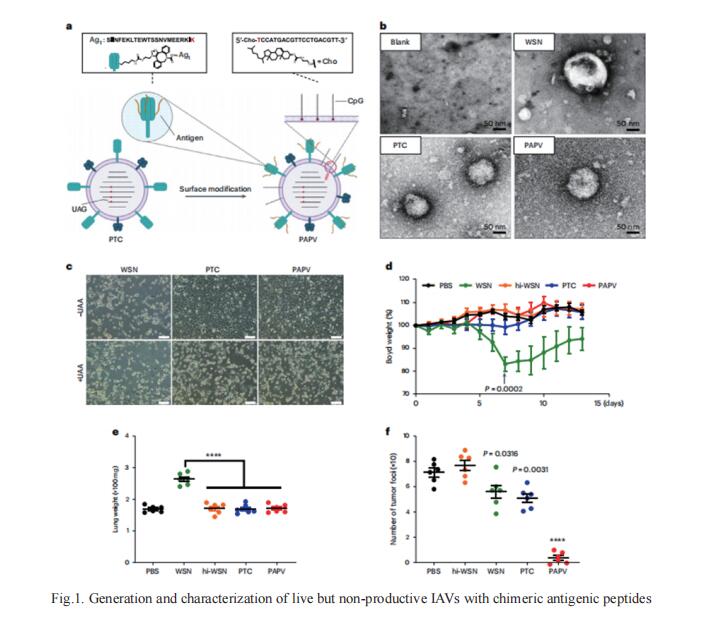
Certain bacterial colonists induce a highly specific T cell response. However, the functional properties of colonist-induced T cells are not well defined, limiting the ability to understand anticommensal immunity and harness it therapeutically. Michael A. Fischbach, Department of Microbiology and Immunology, Stanford University School of Medicine, USA., and his team could elicit T cells that were licensed by the commensal immune program but specific for a tumor by expressing tumor antigens in Staphylococcus epidermidis[2]. Upon colonization, engineered S. epidermidis elicits tumor-specific T cells that circulate, infiltrate local and metastatic lesions, and exert cytotoxic activity. Colonist-induced T cells synergize potently with immune checkpoint blockade (Fig.2), slowing down the progression of invasive B16-F10 melanoma. The results imply that it might be possible to engineer antigen expression into other commensal bacterial strains to elicit a wide range of antigen-specific immune cell responses.
3. Cytokine-armed dendritic cell progenitors for antigen-agnostic cancer immunotherapy
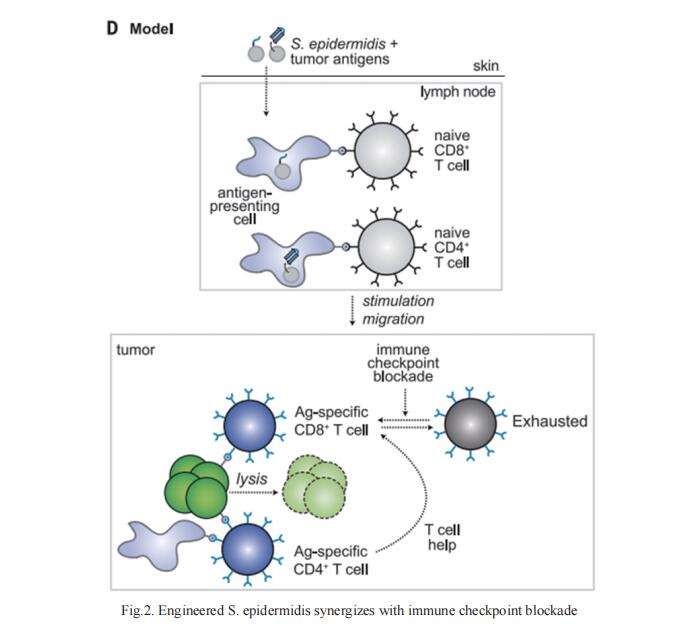
DCs are antigen-presenting myeloid cells that regulate T cell activation, trafcking and function. Monocyte-derived DCs pulsed with tumor antigens have been tested extensively for therapeutic vaccination in cancer, with mixed clinical results. Michele De Palma, School of Life Sciences, Swiss Federal Institute of Technology in Lausanne (EPFL), Switzerland, and his team present a cell-therapy platform based on mouse or human DC progenitors (DCPs) engineered to produce two immunostimulatory cytokines, IL-12 and FLT3L[3]. Cytokine-armed DCPs diferentiated into conventional type-I DCs (cDC1) and suppressed tumor growth, without the need for antigen loading or myeloablative host conditioning. Tumor response involved synergy between IL-12 and FLT3L and was associated with natural killer and T cell infltration and activation, M1-like macrophage programming and ischemic tumor necrosis. Antitumor immunity was dependent on endogenous cDC1 expansion and interferon-γ signaling but did not require CD8+ T cell cytotoxicity. Cytokine-armed DCPs can enhance the cancer efficacy of chemotherapy, anti-PD-1 monoclonal antibody, and GD2-targeting CAR-T cell therapy (Fig.3), demonstrating its potential in combination therapy.
References
[1]Ji D, Zhang Y, Sun J, et al. An engineered influenza virus to deliver antigens for lung cancer vaccination [J]. Nat Biotechnol. 2023. doi:10.1038/s41587-023-01796-7. (IF=46.9)
[2]Chen YE, Bousbaine D, Veinbachs A, et al. Engineered skin bacteria induce antitumor T cell responses against melanoma [J]. Science. 2023;380(6641):203-210. (IF=56.9)
[3]Ghasemi A, Martinez-Usatorre A, Li L, et al. Cytokine-armed dendritic cell progenitors for antigen-agnostic cancer immunotherapy [J]. Nat Cancer. 2023. doi:10.1038/s43018-023-00668-y. (IF=22.7)
Cloud-Clone not only provides a variety of tumor experimental animal models, including tumor transplantation animal models, spontaneous tumor animal models, induced tumor animal models, tumor metastasis animal models, covering common tumor research. We also have various cancer detection indicators, the above-mentioned OVA, IL-12, FLT3L, IFN-γ, PD-1 and other related products, which can help scientific researchers to conduct cancer-related research.