Packages (Simulation)

Reagent Preparation
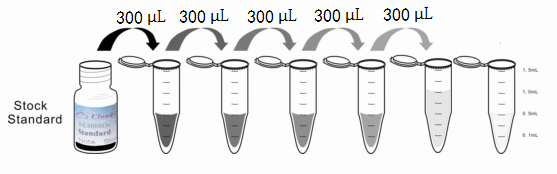
Image (I)
Image (II)
Certificate


Multiplex Assay Kit for Placenta Growth Factor (PLGF) ,etc. by FLIA (Flow Luminescence Immunoassay)
PlGF2; PGF; PGFL; Placental Growth Factor-Like; Vascular Endothelial Growth Factor-Related Protein
(Note: Up to 8-plex in one testing reaction)
- Product No.LMA114Bo
- Organism SpeciesBos taurus; Bovine (Cattle) Same name, Different species.
- Sample TypeSerum, plasma, tissue homogenates, cell lysates, cell culture supernates and other biological fluids
- Test MethodDouble-antibody Sandwich
- Assay Length3.5h
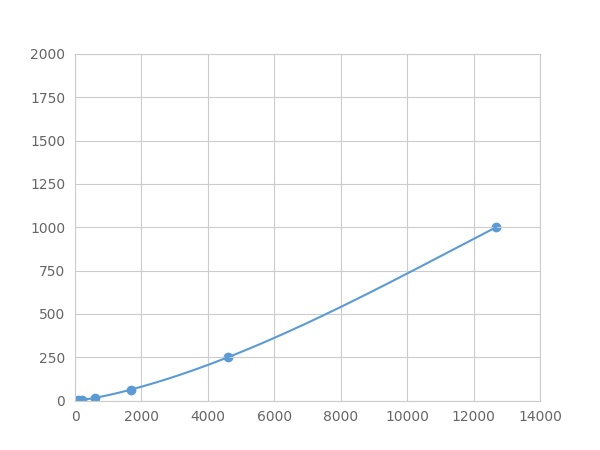
- Detection Range0.98-1000pg/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 0.327 pg/mL.
- DownloadInstruction Manual
- UOM 8Plex 7Plex 6Plex 5Plex 4Plex 3Plex 2Plex1Plex
- FOB
US$ 472
US$ 490
US$ 517
US$ 553
US$ 590
US$ 644
US$ 726
US$ 907
Add to Price Calculator
Result
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Placenta Growth Factor (PLGF) ,etc. by FLIA (Flow Luminescence Immunoassay).
No significant cross-reactivity or interference between Placenta Growth Factor (PLGF) ,etc. by FLIA (Flow Luminescence Immunoassay) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Placenta Growth Factor (PLGF) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Placenta Growth Factor (PLGF) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 87-105 | 96 |
| EDTA plasma(n=5) | 78-91 | 85 |
| heparin plasma(n=5) | 94-102 | 98 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Placenta Growth Factor (PLGF) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Placenta Growth Factor (PLGF) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Placenta Growth Factor (PLGF) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 93-102% | 85-92% | 91-105% | 80-96% |
| EDTA plasma(n=5) | 82-98% | 90-98% | 79-93% | 86-93% |
| heparin plasma(n=5) | 98-105% | 82-96% | 96-105% | 95-105% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:PLGF) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
GIVEAWAYS
INCREMENT SERVICES
| Magazine | Citations |
| Microvascular Research | Mutant hypoxia inducible factor-1α improves angiogenesis and tissue perfusion in ischemic rabbit skeletal muscle PubMed: 20937289 |
| Oncogene | Elevated PLGF contributes to small-cell lung cancer brain metastasis PubMed: 22797069 |
| ?eLife | Photoreceptor avascular privilege is shielded by soluble VEGF receptor-1 PubMed: PMC3687373 |
| International Journal of Nanomedicine | Sustained dual release of placental growth factor-2 and bone morphogenic protein-2 from heparin-based nanocomplexes for direct osteogenesis Pubmed:27042064 |
| The Journal of Maternal-Fetal & Neonatal Medicine | Serum angiogenic profile in abnormal placentation Pubmed:26863111 |
| Fertility and Sterility | Administration of atosiban in patients with endometriosis undergoing frozenâthawed embryo transfer: a prospective, randomized study Pubmed:27143518 |
| Basic & Clinical Pharmacology & Toxicology | Placental Growth Factor Triggers Epithelial-to-Mesenchymal Transition-like Changes in Rat Type II Alveolar Epithelial Cells: Activation of Nuclear Factor κB Signalling Pathway doi:10.1111 |
| Life Sci. | Placenta growth factor contributes to cell apoptosis and epithelial-to-mesenchymal transition in the hyperoxia-induced acute lung injury Pubmed:27211521 |
| Int Immunopharmacol | Knockdown of placental growth factor (PLGF) mitigates hyperoxia-induced acute lung injury in neonatal rats: Suppressive effects on NFκB signaling pathway Pubmed:27280587 |
| Journal of Maternal-Fetal & Neonatal Medicine | Relationship between elevated serum level of placental growth factor and status of gestational diabetes mellitus Pubmed: 30935303 |
| Children (Basel) | sFlt-1/PlGF Ratio in Prediction of Short-Term Neonatal Outcome of Small for Gestational Age Neonates 34438609 |
| Dose-Response: An International Journal | Puerarin Reduces Radiation-Induced Vascular Endothelial Cell Damage Via miR-34a/Placental Growth Factor Pubmed:35110976 |
| Catalog No. | Related products for research use of Bos taurus; Bovine (Cattle) Organism species | Applications (RESEARCH USE ONLY!) |
| RPA114Bo01 | Recombinant Placenta Growth Factor (PLGF) | Positive Control; Immunogen; SDS-PAGE; WB. |
| PAA114Bo01 | Polyclonal Antibody to Placenta Growth Factor (PLGF) | WB; IHC; ICC; IP. |
| SEA114Bo | ELISA Kit for Placenta Growth Factor (PLGF) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| LMA114Bo | Multiplex Assay Kit for Placenta Growth Factor (PLGF) ,etc. by FLIA (Flow Luminescence Immunoassay) | FLIA Kit for Antigen Detection. |