Packages (Simulation)

Reagent Preparation
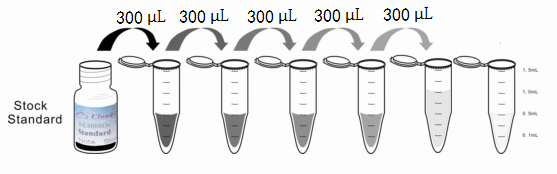
Image (I)
Image (II)
Certificate


Multiplex Assay Kit for Matrix Metalloproteinase 7 (MMP7) ,etc. by FLIA (Flow Luminescence Immunoassay)
MPSL1; MAT; PUMP-1; Matrilysin Uterine; Matrilysin; Matrin; Pump-1 Protease; Uterine Metalloproteinase
(Note: Up to 8-plex in one testing reaction)
- Product No.LMA102Eq
- Organism SpeciesEquus caballus; Equine (Horse) Same name, Different species.
- Sample TypeSerum, plasma and other biological fluids.
- Test MethodDouble-antibody Sandwich
- Assay Length3.5h
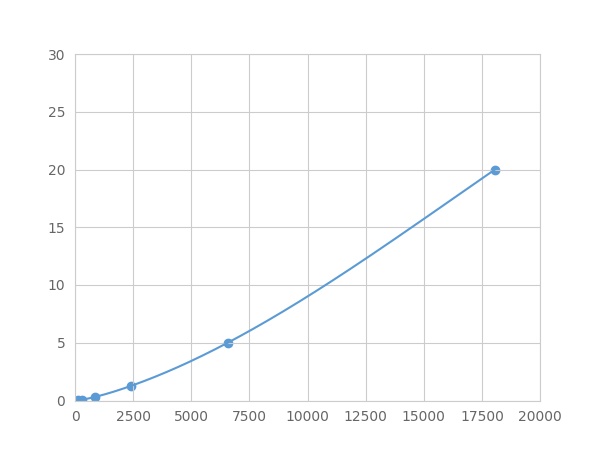
- Detection Range0.02-20ng/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 0.007 ng/mL.
- DownloadInstruction Manual
- UOM 8Plex 7Plex 6Plex 5Plex 4Plex 3Plex 2Plex1Plex
- FOB
US$ 483
US$ 502
US$ 530
US$ 567
US$ 604
US$ 660
US$ 743
US$ 929
Add to Price Calculator
Result
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Matrix Metalloproteinase 7 (MMP7) ,etc. by FLIA (Flow Luminescence Immunoassay).
No significant cross-reactivity or interference between Matrix Metalloproteinase 7 (MMP7) ,etc. by FLIA (Flow Luminescence Immunoassay) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Matrix Metalloproteinase 7 (MMP7) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Matrix Metalloproteinase 7 (MMP7) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 79-98 | 87 |
| EDTA plasma(n=5) | 92-101 | 96 |
| heparin plasma(n=5) | 79-94 | 85 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Matrix Metalloproteinase 7 (MMP7) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Matrix Metalloproteinase 7 (MMP7) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Matrix Metalloproteinase 7 (MMP7) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 82-105% | 86-104% | 83-101% | 79-91% |
| EDTA plasma(n=5) | 87-99% | 90-98% | 98-105% | 91-105% |
| heparin plasma(n=5) | 84-93% | 97-105% | 88-97% | 90-97% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:MMP7) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
GIVEAWAYS
INCREMENT SERVICES
| Magazine | Citations |
| British Journal of Pharmacology | A novel, orally active LPA1 receptor antagonist inhibits lung fibrosis in the mouse bleomycin model PubMed: 20649573 |
| Neuroimmunomodulation | Upregulated Expression of Matrix Metalloproteinases and Tissue Inhibitors of Matrix Metalloproteinases in BALB/c Mouse Brain Challenged with Japanese Encephalitis Virus Pubmed: source |
| PLoS ONE | A Combinatorial Relative Mass Value Evaluation of Endogenous Bioactive Proteins in Three-Dimensional Cultured Nucleus Pulposus Cells of Herniated Intervertebral Discs: Identification of Potential Target Proteins for Gene Therapeutic Approaches Plosone: Source |
| Thromb Haemost. | Analysis of the expression of nine secreted matrix metalloproteinases and their endogenous inhibitors in the brain of mice subjected to ischaemic stroke Pubmed:24671655 |
| Histol Histopathol | Estrogen-deficient osteoporosis enhances the recruitment and activity of osteoclasts by breast cancer cells PubMed: 26254457 |
| VirusDisease | Circulating levels of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases during Japanese encephalitis virus infection Pubmed:26925446 |
| Am J Physiol Lung Cell Mol Physiol | Systematic phenotyping and correlation of biomarkers with lung function and histology in lung fibrosis Pubmed:26993522 |
| Cellular Physiology and Biochemistry | The MiR-495/Annexin A3/P53 Axis Inhibits the Invasion and EMT of Colorectal Cancer Cells pubmed:29224019 |
| s-space | Thyroid disrupting effects and associated mechanisms of TCPP in GH3 cell line and zebrafish (Danio rerio) larva and adult 10371/137708 |
| Science Translational Medicine | Large-scale proteomics identifies MMP-7 as a sentinel of epithelial injury and of biliary atresia. pubmed:29167395 |
| Hepatology | Diagnostic Accuracy of Serum Matrix Metalloproteinase‐7 for Biliary Atresia Pubmed: 30153340 |
| Journal of Pediatrics | Quantification of Serum Matrix Metallopeptide 7 Levels May Assist in the Diagnosis and Predict the Outcome for Patients with Biliary Atresia Pubmed: 30853207 |
| Pediatrics | Serum MMP-7 in the Diagnosis of Biliary Atresia Pubmed: 31604829 |
| Biomed Pharmacother | Human umbilical cord blood mononuclear cells protect against renal tubulointerstitial fibrosis in cisplatin-treated rats Pubmed: 31710895 |
| LABORATORY INVESTIGATION | The synthetic toxin biliatresone causes biliary atresia in mice Pubmed: 32681026 |
| diagnostics | Interstitial Score and Concentrations of IL-4R¦Á, PAR-2, and MMP-7 in Bronchoalveolar Lavage Fluid Could Be Useful Markers for Distinguishing Idiopathic Interstitial?¡ 33924683 |
| Cancer Cell Int | A novel strategy to identify candidate diagnostic and prognostic biomarkers for gastric cancer 34215253 |
| Catalog No. | Related products for research use of Equus caballus; Equine (Horse) Organism species | Applications (RESEARCH USE ONLY!) |
| RPA102Eq01 | Recombinant Matrix Metalloproteinase 7 (MMP7) | Positive Control; Immunogen; SDS-PAGE; WB. |
| PAA102Eq01 | Polyclonal Antibody to Matrix Metalloproteinase 7 (MMP7) | WB; IHC; ICC; IP. |
| MAA102Eq21 | Monoclonal Antibody to Matrix Metalloproteinase 7 (MMP7) | WB; IHC; ICC; IP. |
| SEA102Eq | ELISA Kit for Matrix Metalloproteinase 7 (MMP7) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| SCA102Eq | CLIA Kit for Matrix Metalloproteinase 7 (MMP7) | Chemiluminescent immunoassay for Antigen Detection. |
| LMA102Eq | Multiplex Assay Kit for Matrix Metalloproteinase 7 (MMP7) ,etc. by FLIA (Flow Luminescence Immunoassay) | FLIA Kit for Antigen Detection. |