Packages (Simulation)

Reagent Preparation
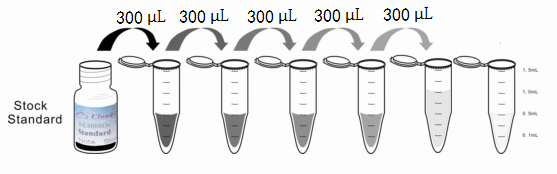
Image (I)
Image (II)
Certificate


Multiplex Assay Kit for Interleukin 1 Receptor Type I (IL1R1) ,etc. by FLIA (Flow Luminescence Immunoassay)
CD121a; CD121-A; IL1-R1; IL1R; IL1RA; P80; CD121 Antigen-Like Family Member A; Interleukin-1 Receptor Alpha
(Note: Up to 8-plex in one testing reaction)
- Product No.LMA066Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- Sample TypeSerum, plasma, tissue homogenates and other biological fluids
- Test MethodDouble-antibody Sandwich
- Assay Length3.5h
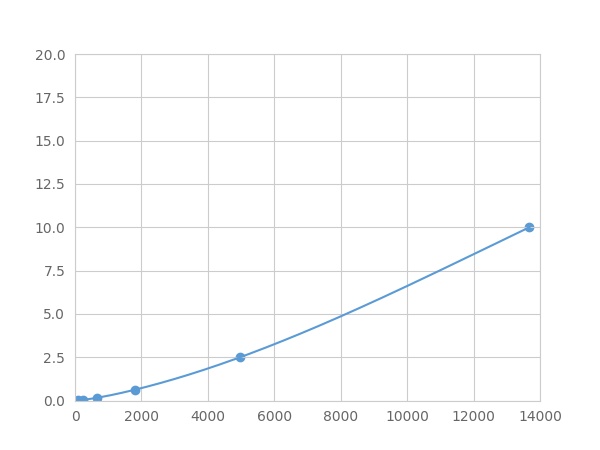
- Detection Range0.01-10ng/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 0.003 ng/mL.
- DownloadInstruction Manual
- UOM 8Plex 7Plex 6Plex 5Plex 4Plex 3Plex 2Plex1Plex
- FOB
US$ 393
US$ 408
US$ 431
US$ 461
US$ 491
US$ 537
US$ 605
US$ 756
Add to Price Calculator
Result
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Interleukin 1 Receptor Type I (IL1R1) ,etc. by FLIA (Flow Luminescence Immunoassay).
No significant cross-reactivity or interference between Interleukin 1 Receptor Type I (IL1R1) ,etc. by FLIA (Flow Luminescence Immunoassay) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Interleukin 1 Receptor Type I (IL1R1) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Interleukin 1 Receptor Type I (IL1R1) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 86-101 | 94 |
| EDTA plasma(n=5) | 78-99 | 91 |
| heparin plasma(n=5) | 96-103 | 101 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Interleukin 1 Receptor Type I (IL1R1) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Interleukin 1 Receptor Type I (IL1R1) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Interleukin 1 Receptor Type I (IL1R1) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 91-99% | 78-91% | 80-89% | 95-103% |
| EDTA plasma(n=5) | 81-97% | 82-92% | 80-97% | 80-88% |
| heparin plasma(n=5) | 95-103% | 93-101% | 91-99% | 85-92% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:IL1R1) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
GIVEAWAYS
INCREMENT SERVICES
| Magazine | Citations |
| Clinical & Experimental Immunology | In vivo extravasation induces expression of IL-1R1 in human neutrophils Wiley: source |
| PLos One | The Gene Expression Analysis of Blood Reveals S100A11 and AQP9 as Potential Biomarkers of Infective Endocarditis Plosone: 0031490 |
| Journal of Clinical Apheresis | Kinetics of the soluble IL-1 receptor type I during treatment with an LCAP filter in patients with inflammatory bowel disease PubMed: 22267087 |
| PLoS ONE | A Combinatorial Relative Mass Value Evaluation of Endogenous Bioactive Proteins in Three-Dimensional Cultured Nucleus Pulposus Cells of Herniated Intervertebral Discs: Identification of Potential Target Proteins for Gene Therapeutic Approaches Plosone: Source |
| 中国当代儿科杂志 | IL1R1 基因多态性与儿童哮喘的相关性 article:13877 |
| STEM CELLS AND DEVELOPMENT | IL-1α regulates osteogenesis and osteoclastic activity of dental follicle cells via JNK and p38 MAPK pathways Pubmed: 33107399 |
| Journal of Integrative Medicine | Salvadora persica extract attenuates cyclophosphamide-induced hepatorenal damage by modulating oxidative stress, inflammation and apoptosis in rats Pubmed:35643766 |