Packages (Simulation)

Reagent Preparation
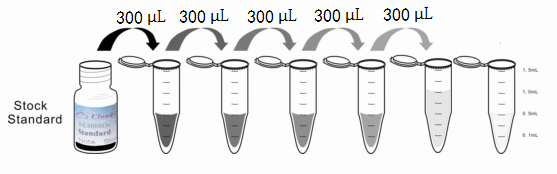
Image (I)
Image (II)
Certificate


Multiplex Assay Kit for Peroxiredoxin 2 (PRDX2) ,etc. by FLIA (Flow Luminescence Immunoassay)
PRP; NKEFB; PRXII; TDPX1; TSA; Thioredoxin-Dependent Peroxide Reductase 1; Thiol-Specific Antioxidant 1; Natural Killer-Enhancing Factor B; Thioredoxin Peroxidase 1; Torin
(Note: Up to 8-plex in one testing reaction)
- Product No.LMF757Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- Sample TypeSerum, plasma, tissue homogenates and other biological fluids
- Test MethodDouble-antibody Sandwich
- Assay Length3.5h
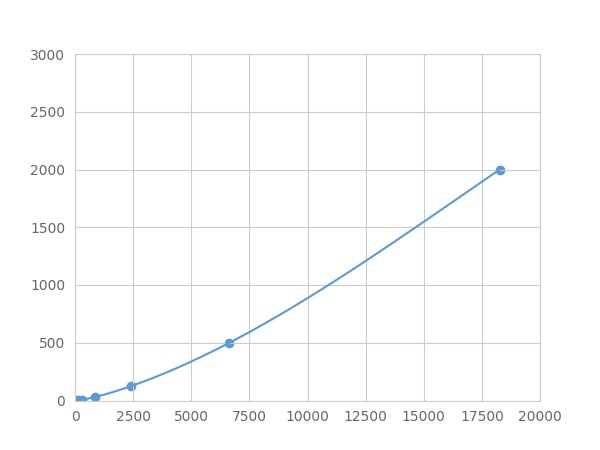
- Detection Range1.95-2000pg/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 0.65 pg/mL.
- DownloadInstruction Manual
- UOM 8Plex 7Plex 6Plex 5Plex 4Plex 3Plex 2Plex1Plex
- FOB
US$ 437
US$ 454
US$ 479
US$ 512
US$ 546
US$ 596
US$ 672
US$ 840
Add to Price Calculator
Result
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Peroxiredoxin 2 (PRDX2) ,etc. by FLIA (Flow Luminescence Immunoassay).
No significant cross-reactivity or interference between Peroxiredoxin 2 (PRDX2) ,etc. by FLIA (Flow Luminescence Immunoassay) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Peroxiredoxin 2 (PRDX2) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Peroxiredoxin 2 (PRDX2) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 93-105 | 101 |
| EDTA plasma(n=5) | 88-101 | 93 |
| heparin plasma(n=5) | 78-101 | 84 |
| sodium citrate plasma(n=5) | 89-103 | 95 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Peroxiredoxin 2 (PRDX2) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Peroxiredoxin 2 (PRDX2) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Peroxiredoxin 2 (PRDX2) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 91-103% | 96-105% | 80-90% | 99-105% |
| EDTA plasma(n=5) | 93-101% | 89-103% | 97-105% | 86-94% |
| heparin plasma(n=5) | 78-95% | 80-95% | 80-96% | 82-103% |
| sodium citrate plasma(n=5) | 86-98% | 94-103% | 83-101% | 80-88% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:PRDX2) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
GIVEAWAYS
INCREMENT SERVICES
| Magazine | Citations |
| Free Radical Research | Thalassemia major patients using iron chelators showed a reduced plasma thioredoxin level and reduced thioredoxin reductase activity, despite elevated oxidative stress Pubmed:25564095 |
| PLoS One | Differences in Proinflammatory Property of Six Subtypes of Peroxiredoxins and Anti-Inflammatory Effect of Ligustilide in Macrophages pubmed:27716839 |
| Experiental And Therapeutic Medicine | Plasma proteins as potential targets of abnormal Savda syndrome in asthma patients treated with unique Uighur prescription pubmed:28672924 |
| Oncotarget | Neuroprotective effect of a novel gastrodin derivative against ischemic brain injury: involvement of peroxiredoxin and TLR4 signaling inhibition pubmed:29207618 |
| European Journal of Applied Physiology | Prolonged forearm ischemia attenuates endothelium-dependent vasodilatation and plasma nitric oxide metabolites in overweight middle-aged men Pubmed:29785503 |
| Journal of Applied Physiology | Montmorency cherry supplementation attenuates vascular dysfunction induced by prolonged forearm occlusion in overweight middle-aged men Pubmed: 30496705 |
| ACS Omega | SOD1 is a Possible Predictor of COVID-19 Progression as Revealed by Plasma Proteomics 34250342 |
| Catalog No. | Related products for research use of Homo sapiens (Human) Organism species | Applications (RESEARCH USE ONLY!) |
| RPF757Hu02 | Recombinant Peroxiredoxin 2 (PRDX2) | Positive Control; Immunogen; SDS-PAGE; WB. |
| RPF757Hu03 | Recombinant Peroxiredoxin 2 (PRDX2) | Positive Control; Immunogen; SDS-PAGE; WB. |
| RPF757Hu01 | Recombinant Peroxiredoxin 2 (PRDX2) | Positive Control; Immunogen; SDS-PAGE; WB. |
| PAF757Hu01 | Polyclonal Antibody to Peroxiredoxin 2 (PRDX2) | WB; IHC; ICC; IP. |
| PAF757Hu03 | Polyclonal Antibody to Peroxiredoxin 2 (PRDX2) | WB; IHC; ICC; IP. |
| PAF757Hu02 | Polyclonal Antibody to Peroxiredoxin 2 (PRDX2) | WB; IHC; ICC; IP. |
| MAF757Hu23 | Monoclonal Antibody to Peroxiredoxin 2 (PRDX2) | WB; IHC; ICC; IP. |
| MAF757Hu21 | Monoclonal Antibody to Peroxiredoxin 2 (PRDX2) | WB |
| WEF757Hu | Wide-range ELISA Kit for Peroxiredoxin 2 (PRDX2) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| SEF757Hu | ELISA Kit for Peroxiredoxin 2 (PRDX2) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| LMF757Hu | Multiplex Assay Kit for Peroxiredoxin 2 (PRDX2) ,etc. by FLIA (Flow Luminescence Immunoassay) | FLIA Kit for Antigen Detection. |