Packages (Simulation)

Reagent Preparation
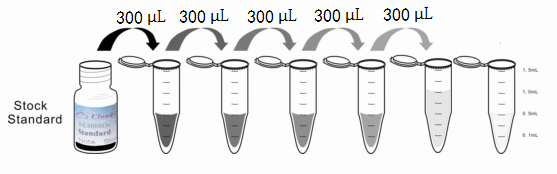
Image (I)
Image (II)
Certificate


Multiplex Assay Kit for Cathepsin K (CTSK) ,etc. by FLIA (Flow Luminescence Immunoassay)
CTS-K; CTS02; CTSO; CTSO1; CTSO2; PKND; PYCD; Pycnodysostosis; Cathepsin O; Cathepsin X
(Note: Up to 8-plex in one testing reaction)
- Product No.LMA267Mu
- Organism SpeciesMus musculus (Mouse) Same name, Different species.
- Sample TypeSerum, plasma, tissue homogenates, cell lysates, cell culture supernates and other biological fluids
- Test MethodDouble-antibody Sandwich
- Assay Length3.5h
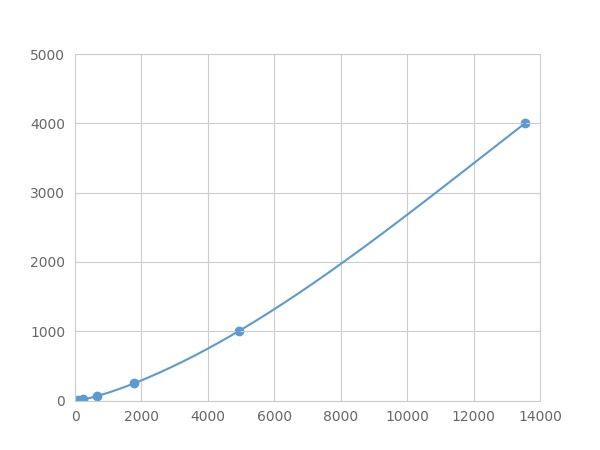
- Detection Range3.91-4000pg/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 1.303 pg/mL.
- DownloadInstruction Manual
- UOM 8Plex 7Plex 6Plex 5Plex 4Plex 3Plex 2Plex1Plex
- FOB
US$ 405
US$ 420
US$ 443
US$ 475
US$ 506
US$ 552
US$ 622
US$ 778
Add to Price Calculator
Result
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Cathepsin K (CTSK) ,etc. by FLIA (Flow Luminescence Immunoassay).
No significant cross-reactivity or interference between Cathepsin K (CTSK) ,etc. by FLIA (Flow Luminescence Immunoassay) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Cathepsin K (CTSK) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Cathepsin K (CTSK) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 94-101 | 97 |
| EDTA plasma(n=5) | 99-105 | 102 |
| heparin plasma(n=5) | 95-102 | 99 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Cathepsin K (CTSK) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Cathepsin K (CTSK) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Cathepsin K (CTSK) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 87-101% | 87-94% | 85-98% | 96-103% |
| EDTA plasma(n=5) | 78-99% | 83-101% | 95-104% | 97-104% |
| heparin plasma(n=5) | 86-94% | 94-103% | 94-105% | 80-93% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:CTSK) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
GIVEAWAYS
INCREMENT SERVICES
| Magazine | Citations |
| Acta Histochemica | MRMT-1 rat breast carcinoma cells and models of bone metastases: improvement of an in vitro system to mimic the in vivo condition. PubMed: S0065128112000773 |
| Atherosclerosis.? | Biochemical and clinical correlation of intraplaque neovascularization using contrast-enhanced ultrasound of the carotid artery Pubmed:24534452 |
| Genet. Mol. Res | Drynaria total flavonoids decrease cathepsin K expression in ovariectomized rats Pubmed:25036175 |
| Age (Dordr) | In vitro method for the screening and monitoring of estrogen-deficiency osteoporosis by targeting peripheral circulating monocytes PubMed: 26250906 |
| Journal of Infectious Diseases | Cathepsin K Contributes to Cavitation and Collagen Turnover in Pulmonary Tuberculosis PubMed: 26416658 |
| PLoS One. | Effect of Vitamin D on Peripheral Blood Mononuclear Cells from Patients with Psoriasis Vulgaris and Psoriatic Arthritis pmc:PMC4822855 |
| Cell Death And Differentiation | TSC1 regulates osteoclast podosome organization and bone resorption through mTORC1 and Rac1/Cdc42 Pubmed:29358671 |
| Indian Journal of Dental Research | Comparative evaluation of cathepsin K levels in gingival crevicular fluid among smoking and nonsmoking patients with chronic periodontitis Pubmed: 30409937 |
| International Journal of Biological Macromolecules | A bioactive exopolysaccharide from marine bacteria Alteromonas sp. PRIM-28 and its role in cell proliferation and wound healing in vitro Pubmed: 30851325 |
| 毕业论文 | Biomarkers of Compromised Implant Fixation |
| neuroscience letters | Compound K induces neurogenesis of neural stem cells in thrombin induced nerve injury through LXRα signaling in mice Pubmed: 32371156 |
| Информационный алгоритм неинвазивной оценки остеорезорбтивного побочного действия глюкокортикостероидов у больных язвенным колитом | |
| Особенности остеоиммунологических аспектов остеорезорбции при периимплантите, хроническом пародонтите и раке альвеолярного отростка и … |
| Catalog No. | Related products for research use of Mus musculus (Mouse) Organism species | Applications (RESEARCH USE ONLY!) |
| RPA267Mu01 | Recombinant Cathepsin K (CTSK) | Positive Control; Immunogen; SDS-PAGE; WB. |
| RPA267Mu02 | Recombinant Cathepsin K (CTSK) | Positive Control; Immunogen; SDS-PAGE; WB. |
| PAA267Mu02 | Polyclonal Antibody to Cathepsin K (CTSK) | WB; IHC; ICC; IP. |
| PAA267Mu01 | Polyclonal Antibody to Cathepsin K (CTSK) | WB; IHC; ICC; IP. |
| SEA267Mu | ELISA Kit for Cathepsin K (CTSK) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| LMA267Mu | Multiplex Assay Kit for Cathepsin K (CTSK) ,etc. by FLIA (Flow Luminescence Immunoassay) | FLIA Kit for Antigen Detection. |