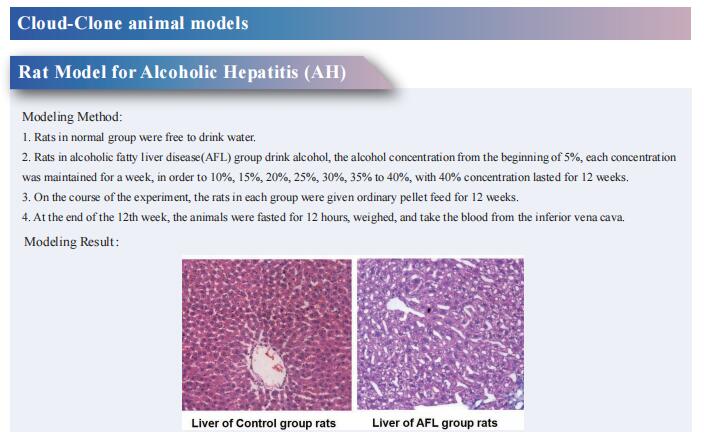
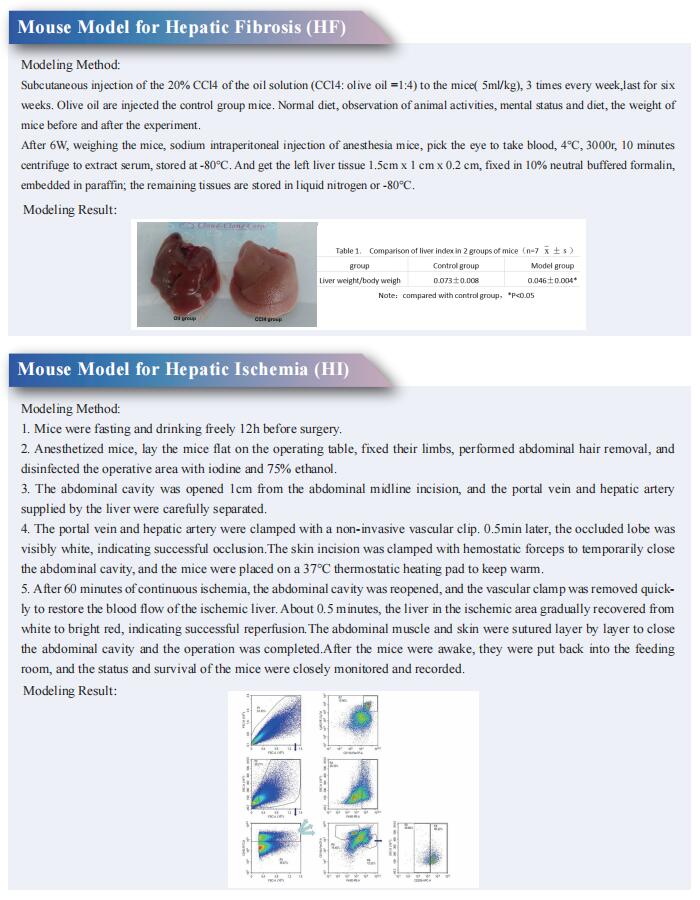
Uncovering the mystery of liver fibrosis, new research may open up effective treatment strategies
Liver fibrosis is the convergent point of various liver diseases, such as alcohol consumption, non-alcoholic steatohepatitis (NASH), viral hepatitis, autoimmune hepatitis, and cholestatic liver disease. The common effect of all of these factors on the liver is the generation of a chronic inflammation resulting in an abnormal wound healing response. The generation of a fibrotic response in the liver gives rise to the accumulation of extracellular matrix (ECM) components, leading to fibrous scar formation. The architecture of the liver is disrupted by the presence of a fibrous scar, which causes hepatocyte loss and the deregulation of the normal functioning of the liver, ultimately resulting in liver failure. When the liver injury is removed, myofibroblasts may undergo apoptosis and inactivation, and therefore clearance of causative etiologies underlying liver fibrosis can slow down the process and lead to fibrosis regression. Despite extensive knowledge on liver fibrosis mechanisms, antifibrotic therapies effective in human have not been developed so far. Recently, multiple studies have reported on liver fibrosis, which may provide assistance in developing new effective treatment strategies.
1. An autocrine signaling circuit in hepatic stellate cells underlies advanced fibrosis in NASH
Advanced hepatic fibrosis, driven by the activation of hepatic stellate cells (HSCs), affects millions world-wide. To identify anti-fibrotic drug targets, Scott L. Friedman, Division of Liver Diseases, Icahn School of Medicine at Mount Sinai, USA, and his team integrated progressive transcriptomic and morphological responses that accompany HSC activation in advanced disease using single nucleus RNA sequencing and tissue clearing in a robust murine NASH model[1]. In advanced fibrosis, they found that an autocrine HSC signaling circuit emerged that was comprised of 68 receptor-ligand interactions conserved between murine and human NASH. These predicted interactions were supported by the parallel appearance of markedly increased direct stellate cell-cell contacts in murine NASH. As proof of principle, pharmacological inhibition of one such autocrine interaction, NTRK3-NTF3, inhibited human HSC activation in culture and reversed advanced murine NASH-fibrosis (Fig.1). This study reveals a new repertoire of antifibrotic drug targets that may be uniquely effective for treating advanced fibrosis.
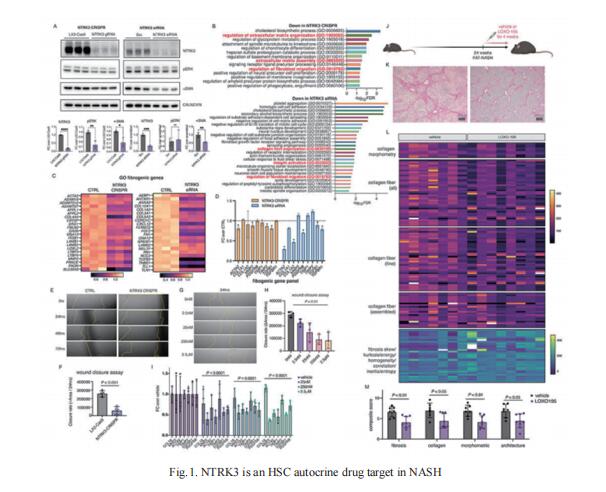
2. Hexokinase 2-mediated gene expression via histone lactylation is required for HSC activation and liver fibrosis
Lactate was implicated in the activation of HSCs. However, the mechanism by which lactate exerts its effect remains elusive. Using RNA-seq and CUT&Tag chromatin profiling, Nissim Hay, Department of Biochemistry and Molecular Genetics, College of Medicine, University of Illinois at Chicago, USA, and his team found that induction of hexokinase 2 (HK2) expression in activated HSCs is required for induced gene expression by histone lactylation but not histone acetylation[2]. Inhibiting histone lactylation by Hk2 deletion or pharmacological inhibition of lactate production diminishes HSC activation, whereas exogenous lactate but not acetate supplementation rescues the activation phenotype. HSC-specific or systemic deletion of HK2 inhibits HSC activation and liver fibrosis in vivo (Fig.2). Therefore, HK2 may be an effective therapeutic target for liver fibrosis.
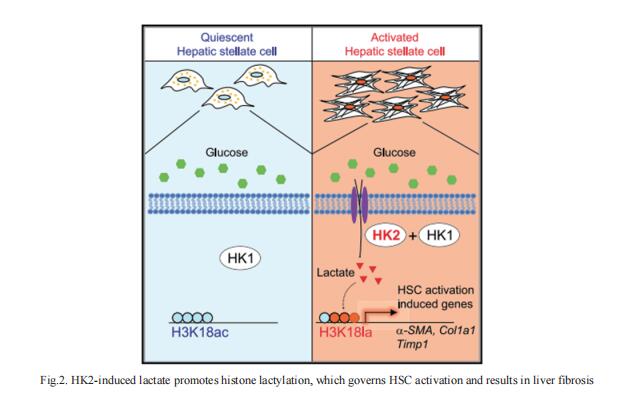
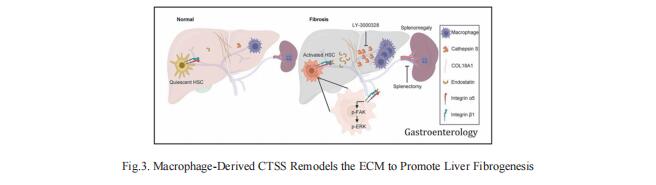
3. Macrophage-Derived Cathepsin S Remodels the ECM to Promote Liver Fibrogenesis
Understanding the molecular mechanisms underlying liver fibrosis progression is relevant for developing new screening and therapeutic strategies. Ping Xu, National Center for Protein Sciences Beijing, China, and his team identified cathepsin S (CTSS) upregulation as a central node for extracellular matrix remodeling in the human fibrotic liver by proteomic screening[3]. Increased serum CTSS levels efficiently predicted liver fibrosis, even at an early stage. Secreted CTSS cleaved collagen 18A1 at its C-terminus, releasing endostatin peptide, which directly bound to and activated hepatic stellate cells via integrin a5b1 signaling, whereas genetic ablation of Ctss remarkably suppressed liver fibrogenesis via endostatin reduction in vivo. Further studies identified macrophages as the main source of hepatic CTSS, and splenectomy effectively attenuated macrophage infiltration and CTSS expression in the fibrotic liver (Fig.3). Pharmacologic inhibition of CTSS ameliorated liver fibrosis progression in the mouse models. These results suggest that CTSS may be a promising target for the diagnosis and treatment of liver fibrosis.
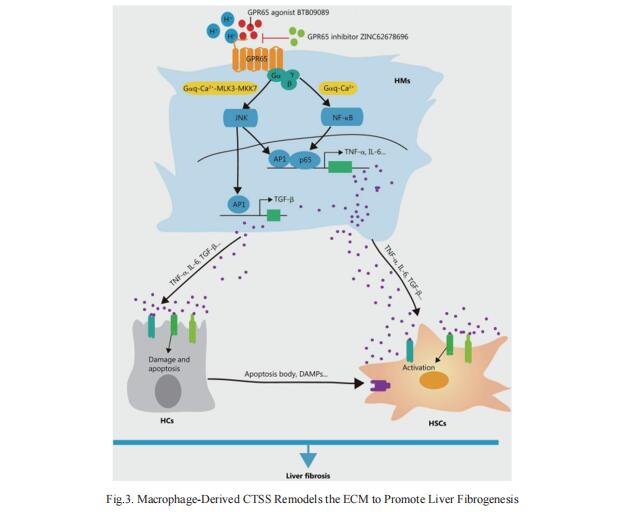
4. Targeting GPR65 alleviates hepatic infammation and fbrosis by suppressing the JNK and NF-κB pathways
G-protein coupled receptors (GPCRs) are recognized as attractive targets for drug therapy. However, it remains poorly understood how GPCRs regulate the progression of liver fibrosis. Wei Hong, Department of Histology and Embryology, School of Basic Medical Sciences, Tianjin Medical University, China, and his team found that knockout of Gpr65 significantly alleviated BDL- and CCl4-induced liver inflammation, injury and fibrosis in vivo, and mouse bone marrow transplantation experiments further demonstrated that the protective effect of Gpr65 knockout is primarily mediated by bone marrow-derived macrophages[4]. GPR65 overexpression and application of GPR65 endogenous and exogenous agonists enhanced the expression and release of TNF-α, IL-6 and TGF-β, all of which subsequently promoted the activation of HSCs and the damage of hepatocytes (HCs). Mechanistically, GPR65 overexpression, the acidic pH and GPR65 exogenous agonist induced up-regulation of TNF-α and IL-6 via the Gαq-Ca2+-JNK/NF-κB pathways, while promoted the expression of TGF-β through the Gαq-Ca2+-MLK3-MKK7-JNK pathway (Fig.4). Notably, pharmacological GPR65 inhibition retarded the development of inflammation, HCs injury and fibrosis in vivo. Thus, targeting GPR65 could be an effective therapeutic strategy for the prevention of liver fibrosis.
References
[1]Wang S, Li K, Pickholz E, et al. An autocrine signaling circuit in hepatic stellate cells underlies advanced fibrosis in nonalcoholic steatohepatitis. Sci Transl Med. 2023;15(677):eadd3949. (IF=17.1)
[2]Rho H, Terry AR, Chronis C, Hay N. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell Metab. 2023;35(8):1406-1423.e8. (IF=29.0)
[3]Zuo T, Xie Q, Liu J, et al. Macrophage-Derived Cathepsin S Remodels the Extracellular Matrix to Promote Liver Fibrogenesis. Gastroenterology. 2023;165(3):746-761.e16. (IF=29.4)
[4]Zhang K, Zhang MX, Meng XX, et al. Targeting GPR65 alleviates hepatic inflammation and fibrosis by suppressing the JNK and NF-κB pathways. Mil Med Res. 2023;10(1):56. (IF=21.1)
Cloud-Clone not only provides animal models of various liver diseases, including liver fibrosis, liver ischemia, alcoholic liver disease, intrahepatic cholestasis, liver cirrhosis, fatty liver, liver failure, etc., covering common liver diseases. We also have multiple species of primary cell products such as hepatic stellate cells, hepatic sinusoidal endothelial cells, hepatic macrophages, and hepatic mesenchymal stem cells, as well as a variety of commonly used detection indicators for liver diseases and the above-mentioned protein-related products such as NTRK3, NTF3, HK2, CTSS, JNK/NF-κB, which can help scientific researchers to carry out liver disease research.