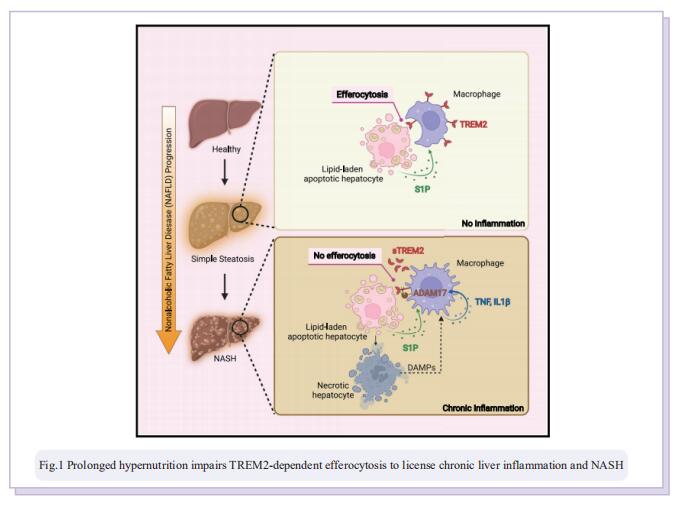
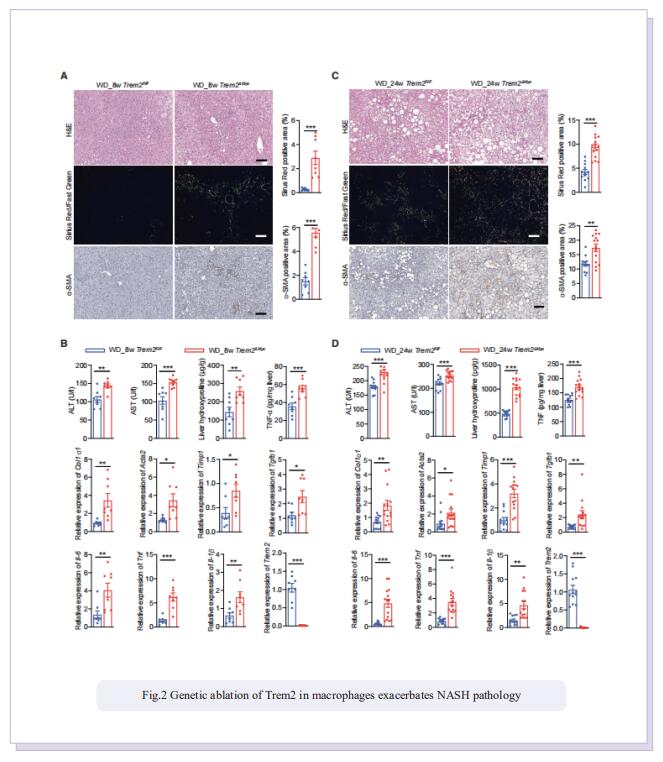
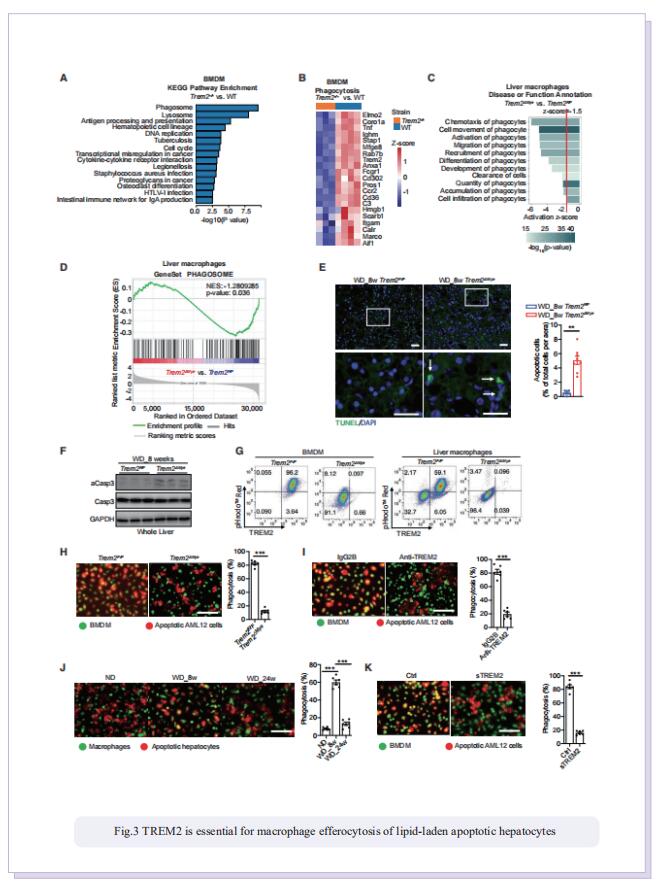
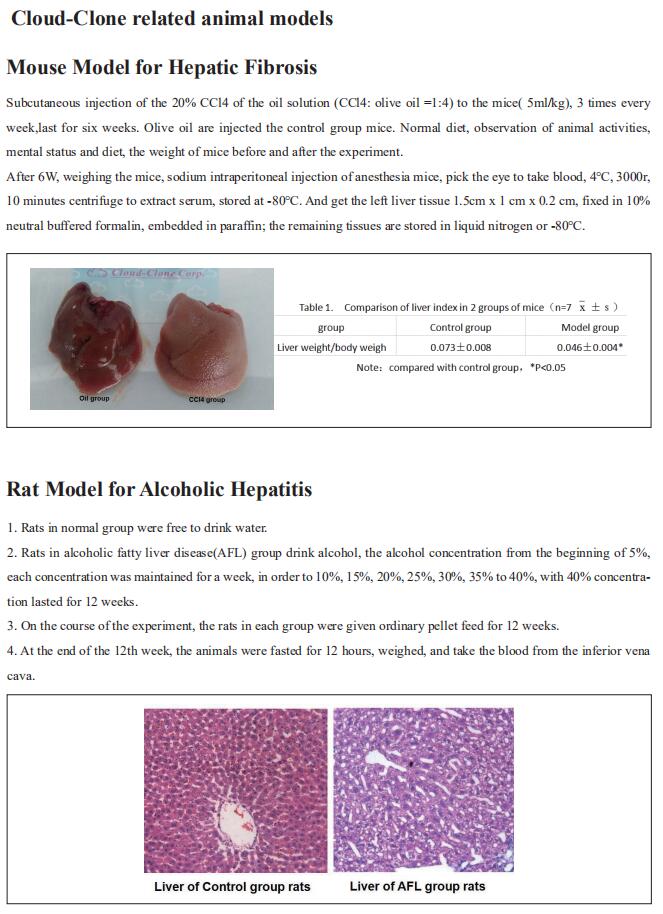
Prolonged hypernutrition impairs TREM2-dependent efferocytosis to license chronic liver inflammation and NASH development
On December 14, 2022, Zhenyu Zhong, Department of Immunology, University of Texas Southwestern Medical Center, USA, and his team published a paper titled “Prolonged hypernutrition impairs TREM2-dependent efferocytosis to license chronic liver inflammation and NASH development” in Immunity. They fdemonstrated that impaired TREM2-dependent efferocytosis is a previously unrecognized key pathogenic event that licenses chronic liver inflammation to ultimately drive simple steatosis transition to nonalcoholic steatohepatitis (NASH).

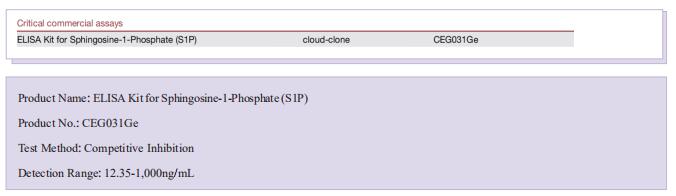
The kits [ELISA Kit for Sphingosine-1-Phosphate (S1P), CEG031Ge] of Cloud-Clone brand was chosed in this article, we are so proud for supporting the reaserchers.
Obesity-induced chronic liver inflammation is a hallmark of nonalcoholic steatohepatitis (NASH)—an aggressive form of nonalcoholic fatty liver disease. However, it remains unclear how such a low-grade, yet persistent, inflammation is sustained in the liver. Here, we show that the macrophage phagocytic receptor TREM2, induced by hepatocyte-derived sphingosine-1-phosphate, was required for efferocytosis of lipid-laden apoptotic hepatocytes and thereby maintained liver immune homeostasis. However, prolonged hypernutrition led to the production of proinflammatory cytokines TNF and IL-1b in the liver to induce TREM2 shedding through ADAM17-dependent proteolytic cleavage. Loss of TREM2 resulted in aberrant accumulation of dying hepatocytes, thereby further augmenting proinflammatory cytokine production. This ultimately precipitated a vicious cycle that licensed chronic inflammation to drive simple steatosis transition to NASH. Therefore, impaired macrophage efferocytosis is a previously unrecognized key pathogenic event that enables chronic liver inflammation in obesity. Blocking TREM2 cleavage to restore efferocytosis may represent an effective strategy to treat NASH.