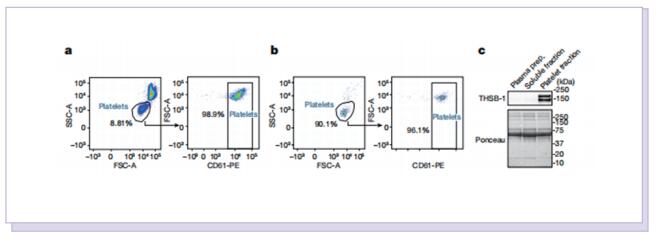
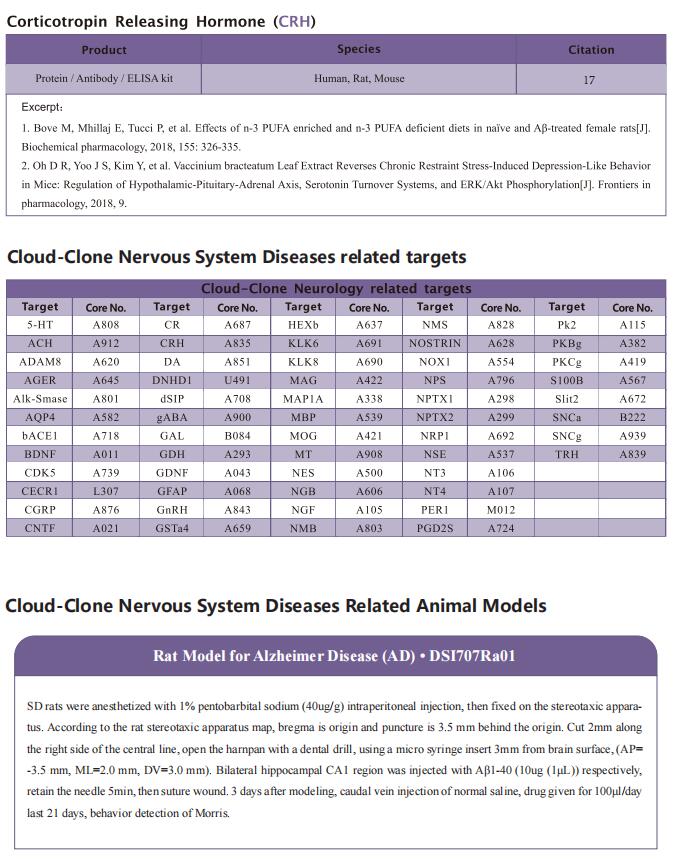
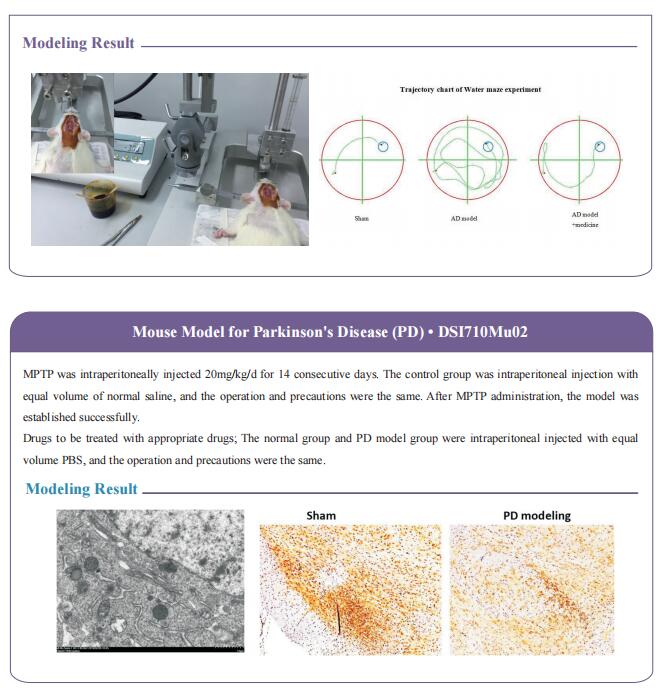
Platelet factors attenuate inflammation and rescue cognition in ageing

Identifying therapeutics to delay, and potentially reverse, age-related cognitive decline is critical in light of the increased incidence of dementia-related disorders forecasted in the growing older population. Systemic rejuvenating interventions—such as heterochronic parabiosis (in which the circulatory systems of young and aged animals are joined)—can reverse age-related impairments in neurogenesis, synaptic plasticity and cognitive function in aged mice. Reports using heterochronic parabiosis led to studies in which systemic administration of blood plasma preparations derived from young or exercised mice was demonstrated to rejuvenate the aged brain. Although the field of rejuvenation research is fast growing, the underlying components in the blood of young animals responsible for reversing age-related brain impairments remain largely unclear.
On August 16, 2023, Saul A. Villeda, Department of Anatomy, University of California San Francisco, USA, and his team published a paper titled “Platelet factors attenuate inflammation and rescue cognition in ageing” in Nature. Their data identified platelet-derived factors as potential therapeutic targets to abate infammation and rescue cognition in old age.

The kit [ELISA Kit for Beta-2-Microglobulin (b2M), SEA260Mu] of Cloud-Clone brand was chosed in this article, we are so proud for supporting the reaserchers.

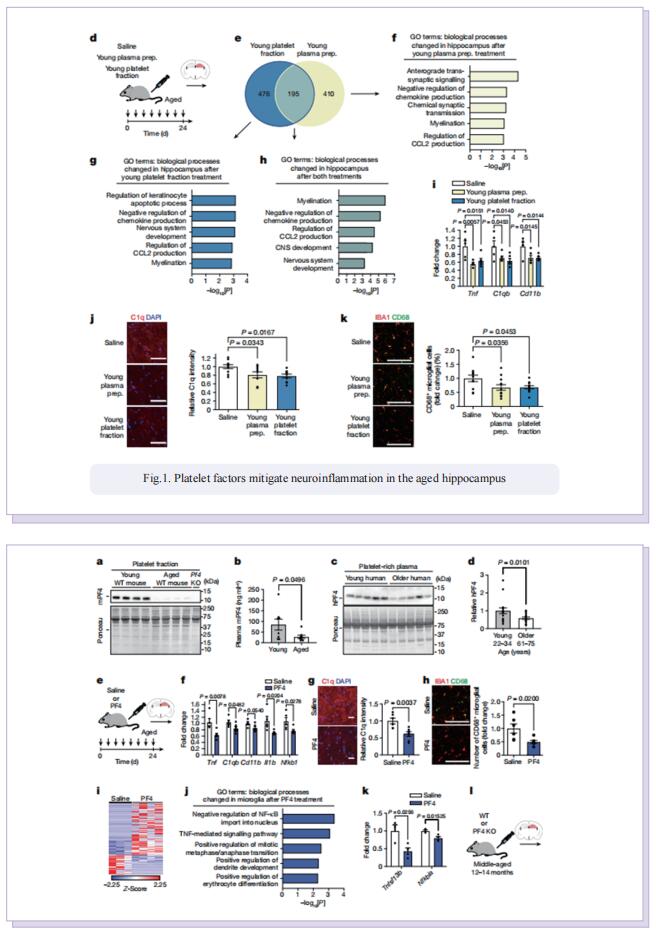
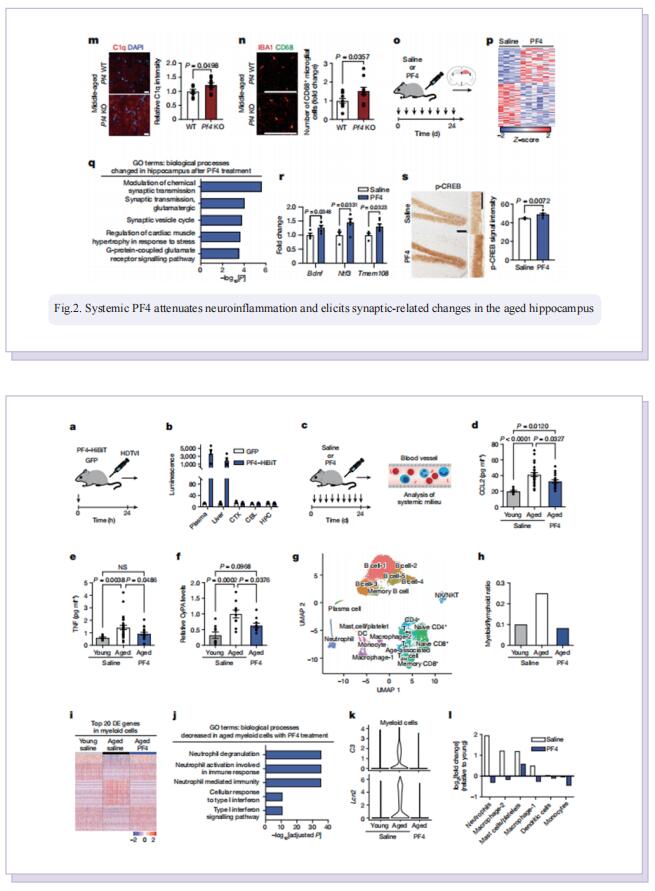
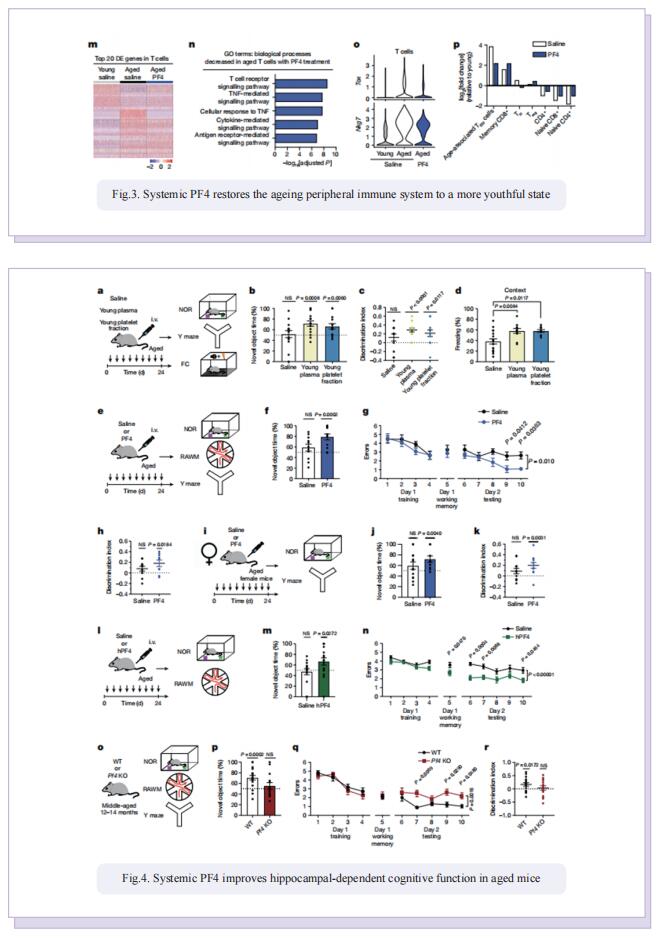
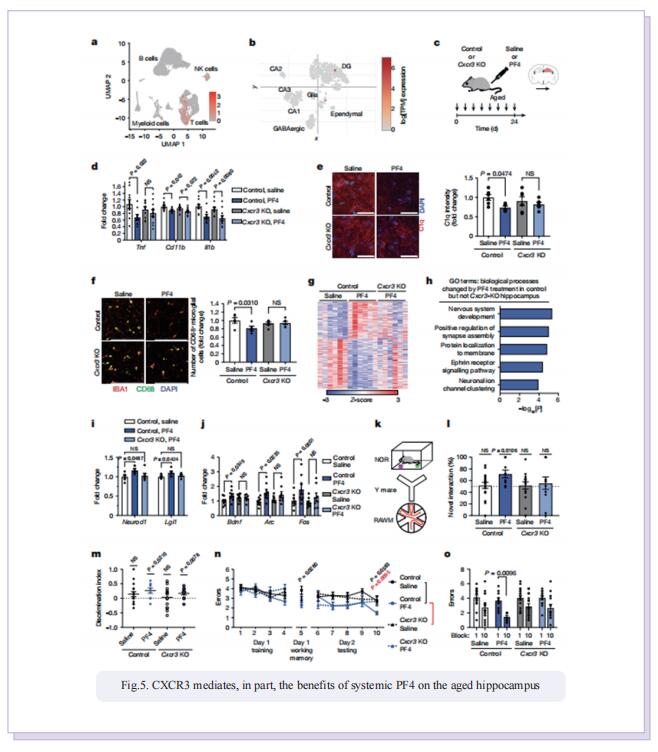
Therefore, the authors sought to define the cellular and molecular mechanisms in the blood of young animals that drive the beneficial effects of systemically administering young blood plasma on the aged brain. They showed that platelet factors transfer the benefts of young blood to the ageing brain. Systemic exposure of aged male mice to a fraction of blood plasma from young mice containing platelets decreased neuroinfammation in the hippocampus at the transcriptional and cellular level and ameliorated hippocampal-dependent cognitive impairments. Systemic exposure of aged male mice to a fraction of blood plasma from young mice containing platelets decreased neuroinfammation in the hippocampus at the transcriptional and cellular level and ameliorated hippocampal-dependent cognitive impairments. Circulating levels of the platelet-derived chemokine platelet factor 4 (PF4) (also known as CXCL4) were elevated in blood plasma preparations of young mice and humans relative to older individuals. Systemic administration of exogenous PF4 attenuated age-related hippocampal neuroinfammation, elicited synaptic-plasticity-related molecular changes and improved cognition in aged mice. They implicate decreased levels of circulating pro-ageing immune factors and restoration of the ageing peripheral immune system in the benefcial efects of systemic PF4 on the aged brain. Mechanistically, they identifed CXCR3 as a chemokine receptor that, in part, mediates the cellular, molecular and cognitive benefts of systemic PF4 on the aged brain.
In summary, the results suggest that maladaptive inflammation and cognitive decline in the aged brain can potentially be reversed by therapeutically leveraging platelet factors to rejuvenate the cellular composition and molecular signature of the ageing peripheral immune system. Given the strong association between increased inflammation and age-related neurodegenerative diseases, such as Alzheimer’s disease, our data further raise the possibility that the beneficial effects of platelet factors may extend more broadly to dementia-related disorders in older people.