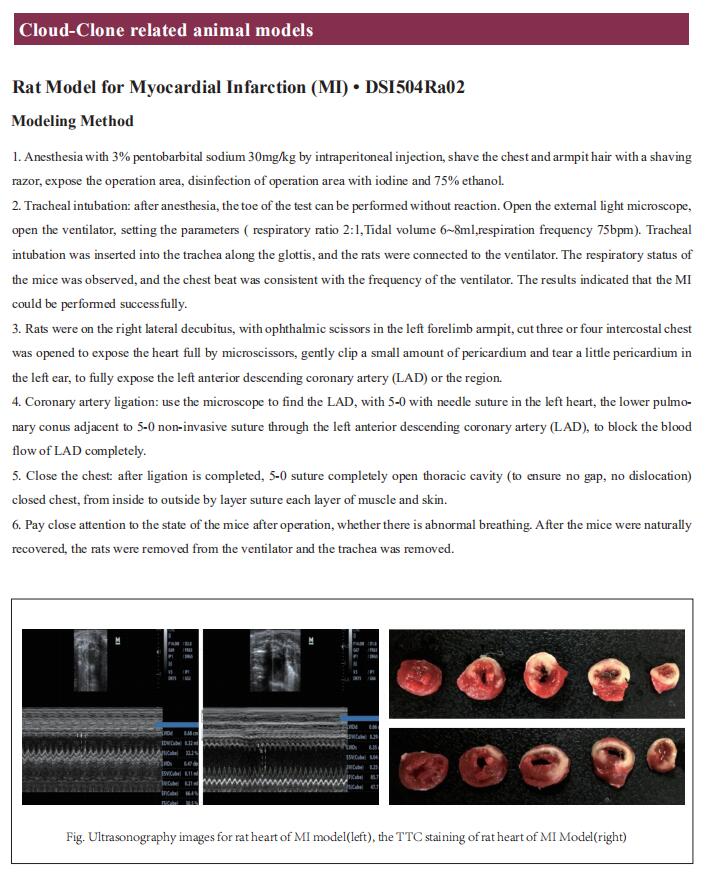
New progress in the study of thrombosis mechanism
Thrombosis - localized clotting of the blood - can occur in the arterial or the venous circulation and has a major medical impact. Acute arterial thrombosis is the proximal cause of most cases of myocardial infarction (heart attack) and of about 80% of strokes, collectively the most common cause of death in the developed world. Venous thromboembolism is the third leading cause of cardiovascular-associated death. The pathogenic changes that occur in the blood vessel wall and in the blood itself resulting in thrombosis are not fully understood. Understanding these processes is crucial for developing safer and more effective antithrombotic drugs.
1. Circadian nuclear receptor Rev-erbα is expressed by platelets and potentiates platelet activation and thrombus formation
The pathological activation of platelets triggers occlusive thrombosis and results in ischaemic events. Such adverse cardiovascular events follow the day/night pattern with a peak in the early morning, potentially related to endogenous circadian clock control of platelet activation. Jun Pu, Division of Cardiology, State Key Laboratory for Oncogenes and Related Genes, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai Cancer Institute, China, and his team reported the presence and functions of circadian nuclear receptor Rev-erbα in human and mouse platelets[1]. Both human and mouse platelet Rev-erbα showed a circadian rhythm that positively correlated with platelet aggregation. Rev-erbα deletion also reduced ferric chloride-induced carotid arterial occlusive thrombosis, prevented collagen/epinephrine-induced pulmonary thromboembolism, and protected against microvascular microthrombi obstruction and infarct expansion in an acute myocardial infarction model. Platelets from Rev-erbα knockout mice exhibited impaired agonist-induced aggregation responses, integrin αIIbβ3 activation, and α-granule release. Consistently, pharmacological inhibition of Rev-erbα by specific antagonists decreasedplatelet activation markers in both mouse and human platelets. Mechanistically, mass spectrometry and co-immunoprecipitation analyses revealed that Rev-erbα potentiated platelet activation via oligophrenin-1-mediated RhoA/ERM pathway (Fig.1). These results suggest that Rev-erbα may serve as a novel therapeutic target for managing thrombosis-based cardiovascular disease.
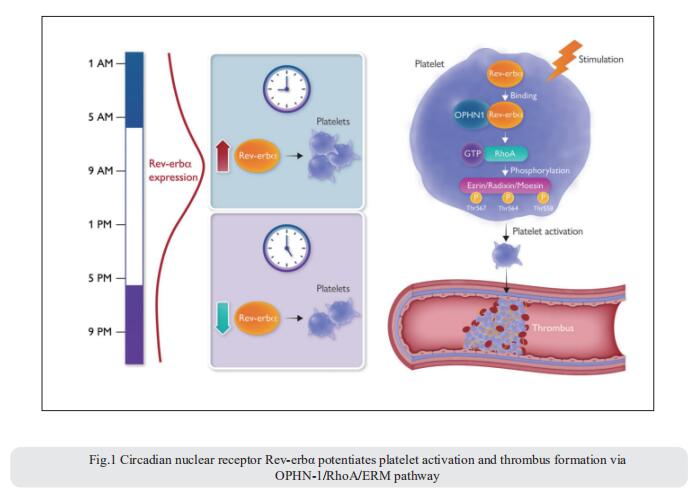
2. KLF2 regulates neutrophil activation and thrombosis
It is widely recognized that inflammation plays a critical role in cardiac hypertrophy and heart failure. Mukesh K. Jain, Case Cardiovascular Research Institute, Case Western Reserve University School of Medicine, Harrington Heart and Vascular Institute, University Hospitals Cleveland Medical Center, USA, and his team found that leukocytes from human subjects and a rodent model of heart failure were characterized by a marked reduction in expression of Klf2 mRNA[2]. Using a mouse model of angiotensin II-induced nonischemic cardiac dysfunction, they showed that neutrophils played an essential role in the pathogenesis and progression of heart failure. Mechanistically, chronic angiotensin II infusion activated a neutrophil KLF2/NETosis pathway that triggered sporadic thrombosis in small myocardial vessels, leading to myocardial hypoxia, cell death, and hypertrophy. Conversely, targeting neutrophils, neutrophil extracellular traps (NETs), or thrombosis ameliorated these pathological changes and preserved cardiac dysfunction. KLF2 regulated neutrophil activation in response to angiotensin II at the molecular level, partly through crosstalk with HIF1 signaling (Fig.2). Taken together, their data implicate neutrophil-mediated immunothrombotic dysregulation as a critical pathogenic mechanism leading to cardiac hypertrophy and heart failure.
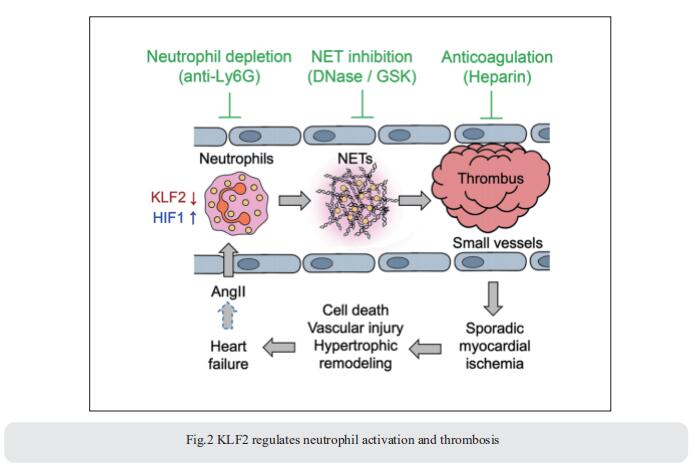
3. Protein tyrosine phosphatase PTPN22 negatively modulates platelet function and thrombus formation
Protein tyrosine phosphatase non-receptor type 22 (PTPN22) is a protein tyrosine phosphatase and negatively regulates T cell signaling. However, whether it is expressed and functions in platelets remains unknown. Jianlin Qiao, Blood Diseases Institute, Xuzhou Medical University, China, and his team showed PTPN22 expression in both human and mouse platelets[3]. Using PTPN22-/- mice, they demonstrated that PTPN22 deficiency significantly shortened tail-bleeding time and accelerated arterial thrombus formation without affecting venous thrombosis and the coagulation factor VIII and IX. PTPN22-deficient platelets exhibited enhanced platelet aggregation, granules secretion, calcium mobilization, lamellipodia formation, spreading and clot retraction. Quantitative phosphoproteomic analysis revealed the significant difference of phosphodiesterase 5A (PDE5A) phosphorylation in PTPN22-deficient platelets compared to wild-type platelets after collagen-related peptide stimulation, which was confirmed by increased PDE5A phosphorylation in CRP-treated PTPN22-deficient platelets, concomitant with reduced cGMP level and VASP phosphorylation. PTPN22 interacted with phosphorylated PDE5A and dephosphorylated it in activated platelets. Furthermore, inhibition of PTPN22 enhanced human platelet aggregation, spreading, clot retraction and increased PDE5A phosphorylation (Fig.3). In conclusion, the study demonstrated a novel role of PTPN22 in platelet function and arterial thrombosis, identifying new potential targets for future prevention of thrombotic or cardiovascular diseases.
References
[1]Shi J, Tong R, Zhou M, et al. Circadian nuclear receptor Rev-erbα is expressed by platelets and potentiates platelet activation and thrombus formation [J]. Eur Heart J. 2022;43(24):2317-2334. (IF=35.855)
[2]Tang X, Wang P, Zhang R, et al. KLF2 regulates neutrophil activation and thrombosis in cardiac hypertrophy and heart failure progression [J]. J Clin Invest. 2022;132(3):e147191. (IF=19.456)
[3]Wang X, Wei G, Ding Y, et al. Protein tyrosine phosphatase PTPN22 negatively modulates platelet function and thrombus formation [J]. Blood. 2022;140(9):1038-1051. (IF=25.476)
Cloud-Clone can not only provide a variety of animal models of cardiovascular and cerebrovascular diseases, covering common diseases such as hypertension, atherosclerosis, myocardial infarction, heart failure, and cerebral ischemia, but also has various detection products of cardiovascular and cerebrovascular diseases related indicators, as well as the above-mentioned OPHN-1, RhoA, KLF2, PTPN22 and other related products, helping researchers to carry out research on thrombosis and related diseases.