New findings on the mechanism of prostate cancer
Prostate cancer (PCa) is the most frequent malignancy in the worldwide male population as well as one of the most common among all the leading cancer-related death causes. Although several men receive an early-stage disease diagnosis and run an indolent course, many cases are characterized by locally advanced or metastatic disease, at the time of diagnosis. Considering the worst course of the advanced disease, a hard challenge for clinicians has been to find a therapeutic approach for these patients in order to improve their prognosis as much as possible. Thus, further exploration of the mechanisms underlying PCa may facilitate the development of more effective therapies.
1. A COP1-GATA2 axis suppresses AR signaling and PCa
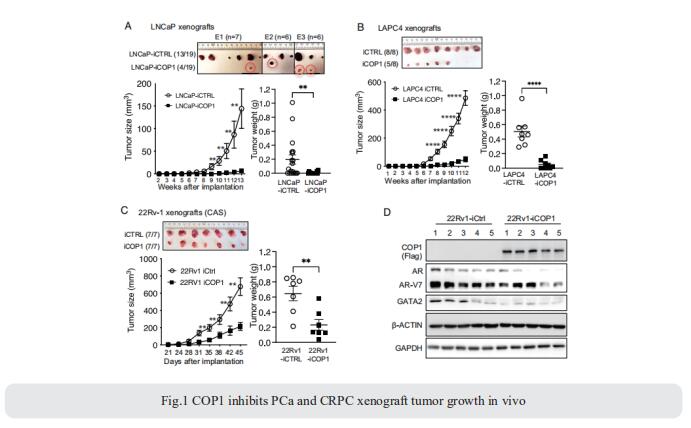
Androgen receptor (AR) signaling is crucial for driving PCa. Androgen deprivation therapy is initially effective in most instances of AR-positive advanced or metastatic PCa. However, patients inevitably develop lethal castration-resistant PCa (CRPC), which is also resistant to the next-generation AR signaling inhibitors. GATA2 is a pioneer transcription factor emerging as a key therapeutic target for PCa because it promotes AR expression and activation. Feng Yang, Department of Molecular and Cellular Biology, Baylor College of Medicine, USA, and his team showed that constitutive photomorphogenesis protein 1 (COP1), an E3 ubiquitin ligase, drives GATA2 ubiquitination at K419/ K424 for degradation[1]. By promoting GATA2 degradation, COP1 inhibits AR expression and activation and represses PCa cell and xenograft growth and castration resistance (Fig.1). Accordingly, GATA2 overexpression or COP1 mutations that disrupt COP1-GATA2 binding block COP1 tumor-suppressing activities. The study concluded that GATA2 is a major COP1 substrate in PCa and that COP1 promotion of GATA2 degradation is a direct mechanism for regulating AR expression and activation, PCa growth, and castration resistance.
2. Gremlin1 regulates lineage plasticity and castration resistance in prostate cancer
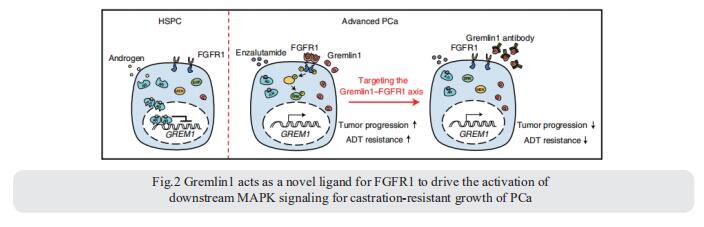
Among the greatest hurdles in clinical management of PCa are the progression to lethal castration-resistant prostate cancer (CRPC) and the lack of suitable targeted therapies for advanced disease. Helen He Zhu, Department of Urology, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, China, and his team identified Gremlin1 as a ligand for fibroblast growth factor receptor1 (FGFR1), which promotes lineage plasticity and drives castration resistance[2]. Importantly, they generated a specific anti-Gremlin1 therapeutic antibody and demonstrate synergistic effect with androgen deprivation therapy (ADT) in CRPC. GREM1 transcription is suppressed by androgen receptor (AR) and released following ADT. We show that Gremlin1 binds to FGFR1 and activates downstream MAPK signaling (Fig.2). Gremlin1 interacts with FGFR1 differently to its canonical ligand FGF1, as revealed through protein structure docking and mutagenesis experiments. Altogether, those data indicate Gremlin1 as a promising candidate therapeutic target for CRPC.
3. HOXB13 suppresses de novo lipogenesis through HDAC3-mediated epigenetic reprogramming in prostate cancer
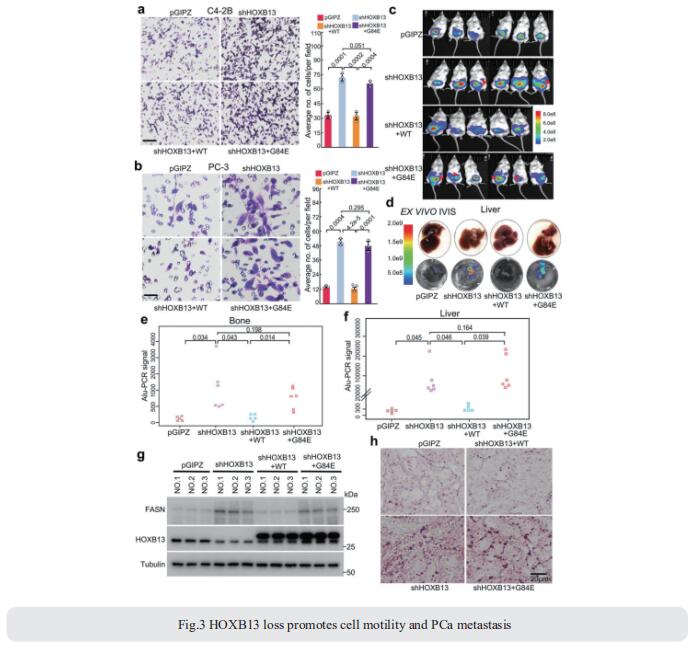
HOXB13, a homeodomain transcription factor, critically regulates androgen receptor (AR) activities and androgen-dependent prostate cancer (PCa) growth. Jindan Yu, Division of Hematology/Oncology, Department of Medicine, Northwestern University Feinberg School of Medicine, USA, and his team reported HOXB13 interaction with histone deacetylase HDAC3, which is disrupted by HOXB13 G84E mutation that has been associated with early-onset PCa[3]. Independently of AR, HOXB13 recruits HDAC3 to lipogenic enhancers to catalyze histone deacetylation and suppress lipogenic regulators such as fatty acid synthase (FASN). HOXB13 loss or G84E mutation leads to lipid accumulation in PCa cells, thereby promoting cell motility and xenograft tumor metastasis (Fig.3), which is mitigated by pharmaceutical inhibition of FASN. In summary, we present evidence that HOXB13 recruits HDAC3 to suppress de novo lipogenesis and inhibit tumor metastasis and that lipogenic pathway inhibitors may be useful to treat HOXB13-low PCa.
4. KMT2C methyltransferase domain regulated INK4A expression suppresses prostate cancer metastasis
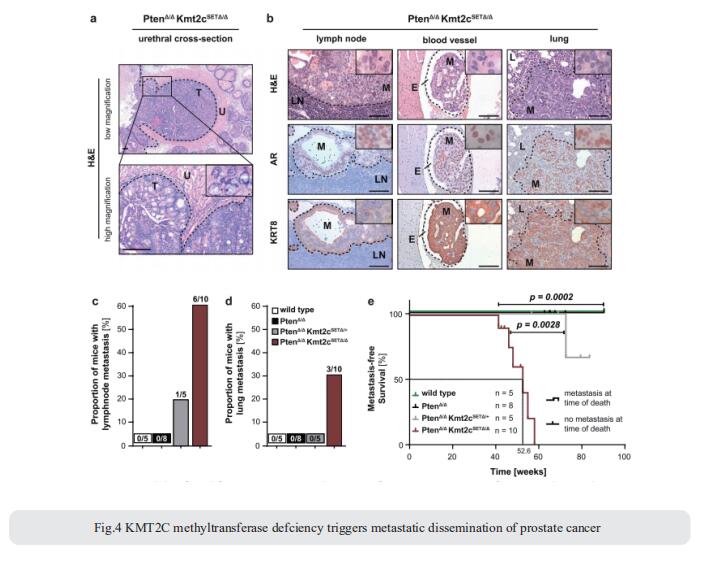
Frequent truncation mutations of the histone lysine N-methyltransferase KMT2C have been detected by whole exome sequencing studies in various cancers, including malignancies of the prostate. To investigate the functional effects of these mutations, Lukas Kenner, Division of Experimental and Translational Pathology, Department of Pathology, Medical University of Vienna, Austria, deleted the C-terminal catalytic core motif of KMT2C specifically in mouse prostate epithelium[4]. They analysed the effect of KMT2C SET domain deletion in a Pten-deficient PCa mouse model in vivo and of truncation mutations of KMT2C in a large number of prostate cancer patients.They showed that impaired KMT2C methyltransferase activity drives proliferation and PIN formation and, when combined with loss of the tumour suppressor PTEN, triggers loss of senescence, metastatic dissemination and dramatically reduces life expectancy (Fig.4). In KMT2C-mutated tumours they show enrichment of proliferative MYC gene signatures and loss of expression of the cell cycle repressor p16INK4A. In addition, they observed a striking reduction in disease-free survival of patients with KMT2C-mutated prostate cancer. The data demonstrate the prognostic signifcance of KMT2C mutation status in prostate cancer patients. Inhibition of the MYC signalling axis may be a viable treatment option for patients with KMT2C truncations and therefore poor prognosis.
References
[1]Shen T, Dong B, Meng Y, et al. A COP1-GATA2 axis suppresses AR signaling and prostate cancer [J]. Proc Natl Acad Sci U S A. 2022;119(43):e2205350119. (IF=12.779)
[2]Cheng C, Wang J, Xu P, et al. Gremlin1 is a therapeutically targetable FGFR1 ligand that regulates lineage plasticity and castration resistance in prostate cancer [J]. Nat Cancer. 2022;3(5):565-580. (IF=23.177)
[3]Lu X, Fong KW, Gritsina G, et al. HOXB13 suppresses de novo lipogenesis through HDAC3-mediated epigenetic reprogramming in prostate cancer [J]. Nat Genet. 2022;54(5):670-683. (IF=41.307)
[4]Limberger T, Schlederer M, Trachtová K, et al. KMT2C methyltransferase domain regulated INK4A expression suppresses prostate cancer metastasis [J]. Mol Cancer. 2022;21(1):89. (IF=41.444)
Cloud-Clone not only provides a variety of tumor experimental animal models, including tumor transplantation animal models, spontaneous tumor animal models, induced tumor animal models, tumor metastasis animal models, etc., covering common tumor research. We also have various cancer detection indicators and the above-mentioned GATA2, Gremlin1, FGFR1, MAPK and other related products, which can help scientific researchers to conduct cancer-related research.