New findings on the mechanism of atopic dermatitis
Atopic dermatitis (AD) is a recurrent, chronic, non-infectious inflammatory dermatosis characterized by persistent itching of the skin. The clinical picture includes eczema-like eruptions, such as erythema, papules, exudative lesions of a specific location, depending on the patient’s age andvarious skin degrees of dryness. The pathophysiology of atopic dermatitis is complex and multifactorial. Its understanding is complicated by the number of synergized factors that influence the disease. The most important are: genetic disorders, a defect in the epidermal barrier, an altered immune response and disturbed microbiological balance of the skin. In addition, environmental factors such as increased exposure to air or food allergens, pollution, infection, and antibiotic use also play a role in the progression of AD pathology. Recently, many literatures have reported AD pathological related research, which may be helpful for AD clinical treatment.
1. Hsa_circ_0004287 inhibits macrophagemediated inflammation in an N6-methyladenosine–dependent manner in atopic dermatitis
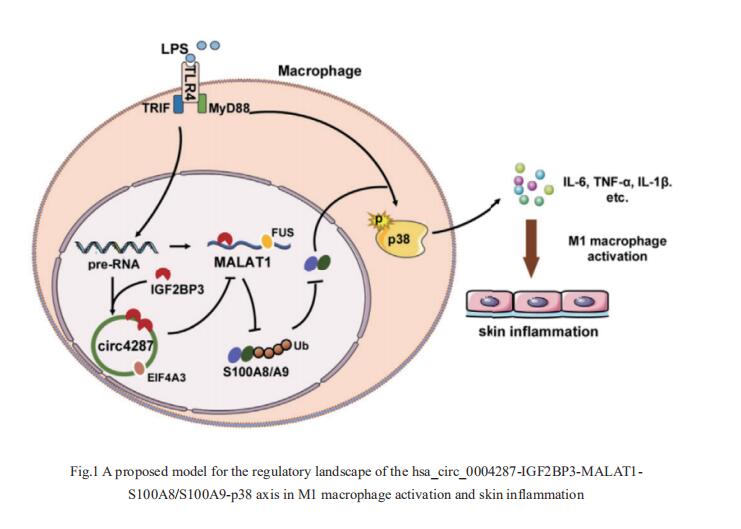
Circular RNA (circRNA) has been implicated in various diseases. Yufeng Zhou, Institute of Pediatrics, Children’s Hospital of Fudan University, China, and hia team sought to determine the differential expression profiles of circRNAs in peripheral blood mononuclear cells between healthy controls and AD patients, and explore the mechanisms underlying the effects of circRNAs on the pathogenesis of AD[1]. They identified a functional unknown circRNA hsa_circ_0004287 from 88750 circRNAs, which was upregulated in peripheral blood mononuclear cells of AD patients, and was mainly expressed by macrophages under inflammatory conditions. Hsa_circ_0004287 inhibited M1 macrophage activation in vitro, and macrophage-specific overexpression of hsa_circ_0004287 alleviated skin inflammation in AD-like mice. Mechanistically, hsa_circ_0004287 reduced the stability of its host gene metastasis associated lung adenocarcinoma transcript 1 (MALAT1) by competitively binding to IGF2BP3 with MALAT1 in an N6-methyladenosine (m6A)-dependent manner(Fig.1). Lower levels of MALAT1 promoted the ubiquitination degradation of S100A8/S100A9, thereby impeding p38/mitogenactivated protein kinase phosphorylation and macrophagemediated inflammation. This study showed that hsa_circ_0004287 inhibits M1 macrophage activation in an m6A-dependent manner in AD, and may serve as a general therapeutic candidate for AD.
2. Human-β-defensin-3 attenuates atopic dermatitis-like inflammation through autophagy activation and the aryl hydrocarbon receptor signaling pathway
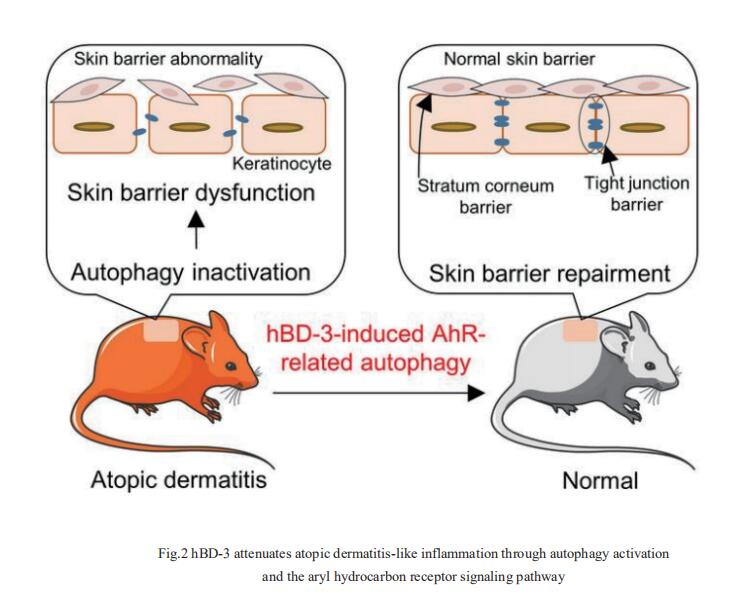
Human β-defensin (hBD)-3 exhibits antimicrobial and immunomodulatory activitie; however, its contribution to autophagy regulation remains unclear, and the role of autophagy in the regulation of the epidermal barrier in atopic dermatitis (AD) is poorly understood. François Niyonsaba, Atopy (Allergy) Research Center, Juntendo University Graduate School of Medicine, Japan, and his team showed that keratinocyte autophagy was restrained in the skin lesions of patients with AD and murine models of AD[2]. hBD-3 alleviated the interleukin-4- and interleukin-13-mediated impairment of the tight junction (TJ) barrier through keratinocyte autophagy activation, which involved aryl hydrocarbon receptor (AhR) signaling(Fig.2). While autophagy deficiency impaired the epidermal barrier and exacerbated inflammation, hBD-3 attenuated skin inflammation and enhanced the TJ barrier in AD. Importantly, hBD-3-mediated improvement of the TJ barrier was abolished in autophagy-deficient AD mice and in AhR-suppressed AD mice, suggesting a role for hBD-3-mediated autophagy in the regulation of the epidermal barrier and inflammation in AD. The study highlighted the role of hBD-3 as a novel autophagy activator in a new approach to the treatment of AD that functions via autophagy activation and uncovered the importance of AhR in hBD-3-mediated autophagy.
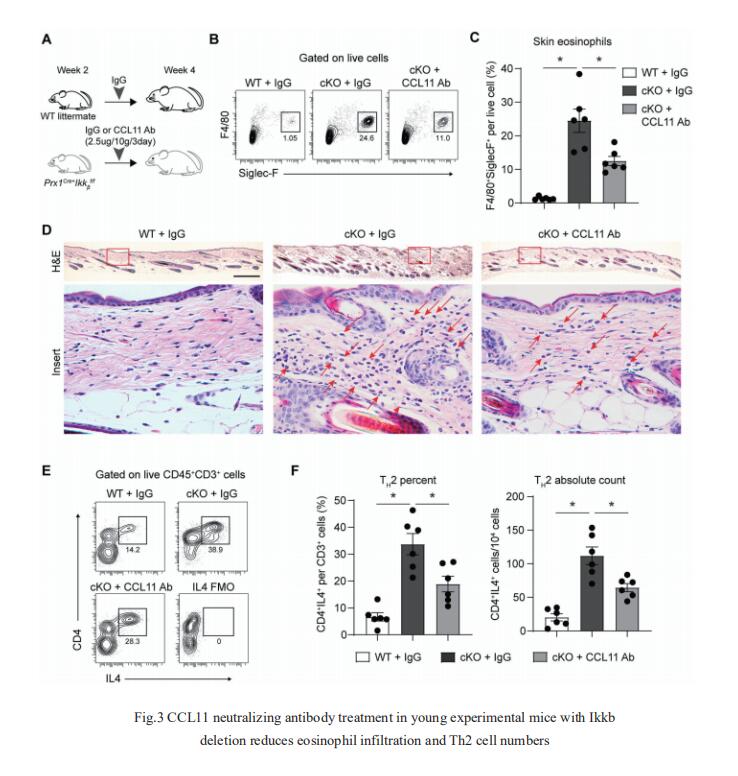
3. NF-kB perturbation reveals unique immunomodulatory functions in Prx1+ fibroblasts that promote development of atopic dermatitis
Skin is composed of diverse cell populations that cooperatively maintain homeostasis. Through analysis of single cell RNA sequencing data via iterative Random Forest Leave One Out Prediction, an explainable artificial intelligence method, JOHN T. SEYKORA, Department of Dermatology, Perelman School of Medicine, University of Pennsylvania, USA, and his team identified an immunoregulatory role for a unique Prx1+ fibroblast subpopulation[3]. Disruption of Ikkb-NF-kB under homeostatic conditions in these fibroblasts paradoxically induced skin inflammation due to the overexpression of C-C Motif Chemokine Ligand 11 (CCL11, or eotaxin-1) characterized by eosinophil infiltration and a subsequent Th2 immune response. Because the inflammatory phenotype resembled that seen in human atopic dermatitis (AD), they examined validated human AD skin samples and found that human AD fibroblasts also over-expressed CCL11 and that perturbation of Ikkb-NFkB in primary human dermal fibroblasts upregulates CCL11. They also demonstrated that monoclonal antibody treatment against CCL11 was effective in reducing the eosinophilia and Th2 inflammation in a mouse model(Fig.3). Taken together, the murine model and human AD specimens point to dysregulated Prx1+ fibroblasts as a previously unrecognized etiologic factor that may contribute to the pathogenesis of AD and suggest targeting CCL11 as a way to treat AD-like skin lesions.
References
[1]Yang L, Fu J, Han X, et al. Hsa_circ_0004287 inhibits macrophage-mediated inflammation in an N6-methyladenosine-dependent manner in atopic dermatitis and psoriasis[J]. J Allergy Clin Immunol. 2022, 149(6):2021-2033. (IF=14.290)
[2]Peng G, Tsukamoto S, Ikutama R, et al. Human-β-defensin-3 attenuates atopic dermatitis-like inflammation through autophagy activation and the aryl hydrocarbon receptor signaling pathway [J]. J Clin Invest. 2022, e156501. (IF=19.456)
[3]Ko KI, Merlet JJ, DerGarabedian BP, et al. NF-κB perturbation reveals unique immunomodulatory functions in Prx1+ fibroblasts that promote development of atopic dermatitis[J]. Sci Transl Med. 2022, 14(630):eabj0324. (IF=19.319)
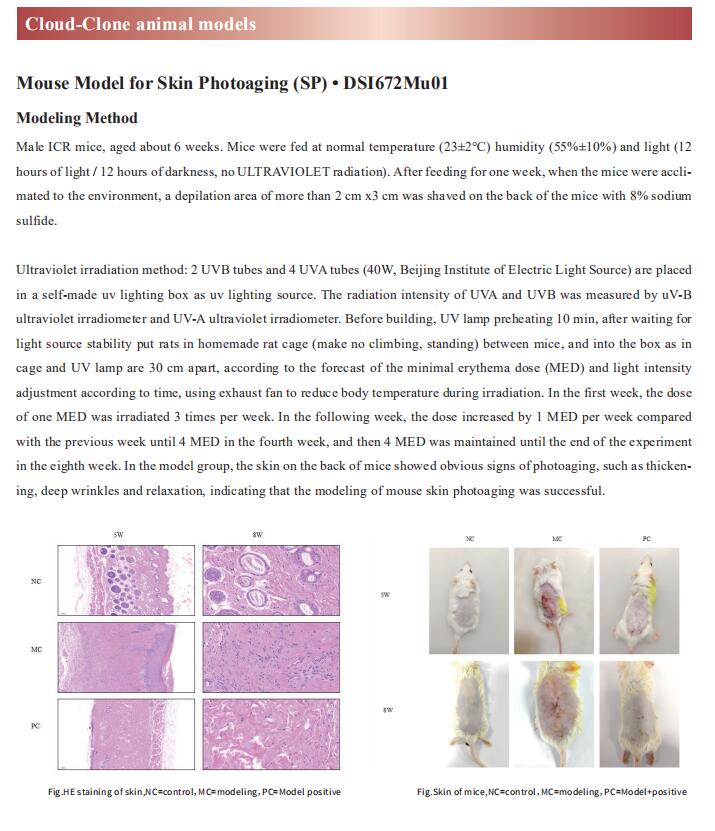
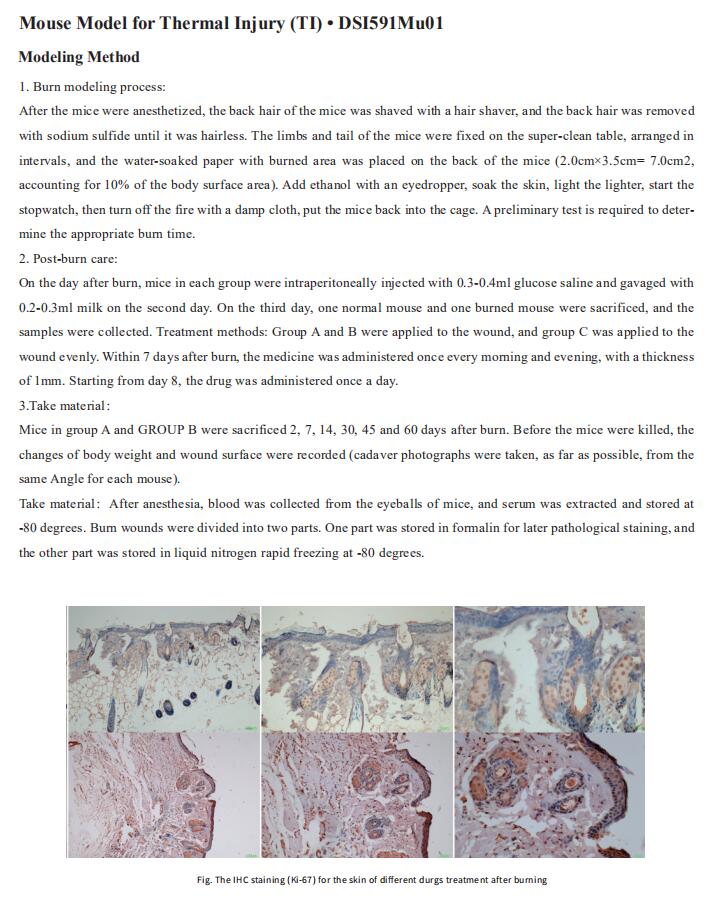
Cloud-Clone can not only provide a variety of skin disease animal models, including psoriasis, skin allergy, skin photoaging, contact dermatitis, scleroderma, etc., covering common skin diseases. We also have various skin disease detection indicators and the above IGF2BP3, HBD-3, CCL11, NF-KB pathway related products, which can help the majority of scientific researchers to carry out skin disease related research.