New findings in the mechanism of multiple sclerosis
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS)characterized by demyelination, axonal damage, and progressive neurologic disability. MS is a disease that affects brain, spinal cord and the optic nerves. The main symptoms include fatigue, tremor, motor dysfunction, nystagmus, numbness, loss of coordination or balance, disturbances in speech and vision, cognitive impairment and acute paralysis, and it is one of the most common neurological (non-traumatic) cause of disability in young adults. The etiology and pathogenesis of MS is complex and remain elusive, although both genetic and environmental factors are involved. Recently, several studies on the pathological mechanism of MS have been reported, which may provide help for the prevention and treatment of MS.
1. MS and Gut microbiome
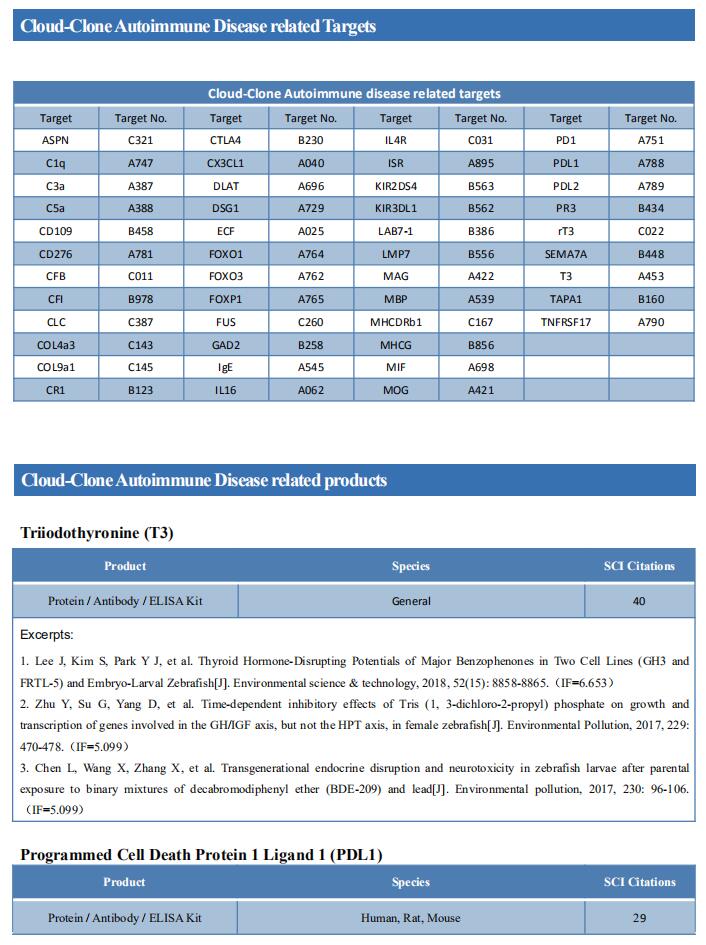
Changes in gut microbiota have been associated with several diseases. The international Multiple Sclerosis Microbiome Study (iMSMS) studied the gut microbiome of 576 MS patients (36% untreated), and genetically unrelated household healthy controls (1,152 total subjects) (Fig.1)[1]. They observed a significantly increased proportion of Akkermansia muciniphila, Ruthenibacterium lactatiformans, Hungatella hathewayi and Eisenbergiella tayi and decreased Faecalibacterium prausnitzii and Blautia species. The phytate degradation pathway was over-represented in untreated MS, while pyruvate-producing carbohydrate metabolism pathways were significantly reduced. The therapeutic activity of interferon-β may in part be associated to upregulation of short chain fatty acid transporters. Distinct microbial networks were observed in untreated MS and healthy controls. These results strongly support specific gut microbiome associations with MS risk, course and progression, and functional changes in response to treatment.
2. MS and Epstein-Barr virus
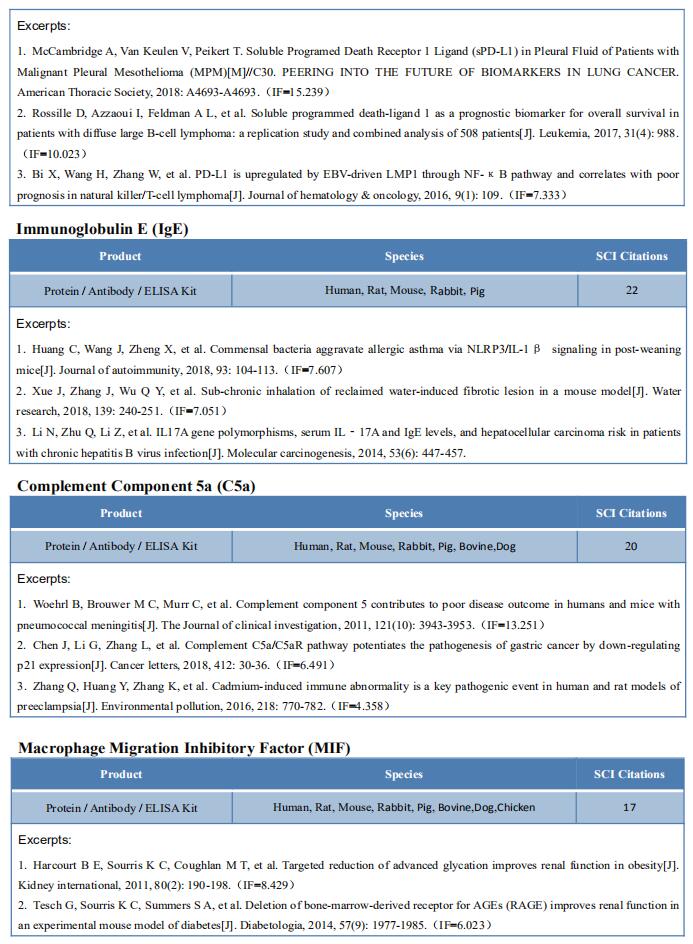
Epstein-Barr virus (EBV) infection has been linked to MS epidemiologically, but its pathological role remains unclear. William H. Robinson, Division of Immunology and Rheumatology, Department of Medicine, Stanford University School of Medicine, USA, and his team demonstrated high-affinity molecular mimicry between the EBV transcription factor EBNA1 and the CNS protein GlialCAM (Fig.2), and provide structural and in-vivo functional evidence for its relevance[2]. A cross-reactive CSF-derived antibody was initially identified by single-cell sequencing of the paired-chain B cell repertoire of MS blood and CSF, followed by protein microarray-based testing of recombinantly expressed CSF-derived antibodies against MS-associated viruses. Sequence analysis, affinity measurements, and the crystal structure of the EBNA1-peptide epitope in complex with the autoreactive Fab fragment allowed for tracking the development of the naïve EBNA1-restricted antibody to a mature EBNA1/GlialCAM cross-reactive antibody. Molecular mimicry is facilitated by a posttranslational modification of GlialCAM. EBNA1 immunization exacerbates the mouse model of MS and anti-EBNA1/GlialCAM antibodies are prevalent in MS patients. The results provide a mechanistic link for the association between MS and EBV, and could guide the development of novel MS therapies.
3. MS and Bone marrow hematopoiesis
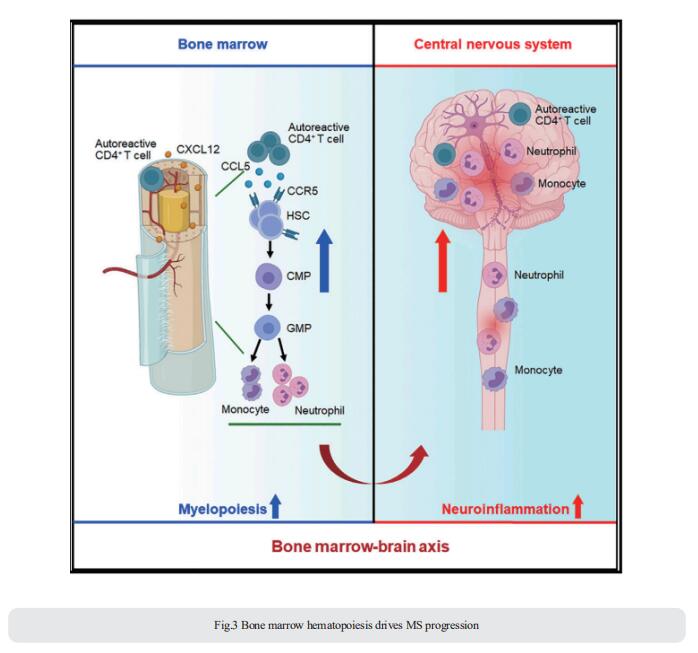
Bone marrow hematopoietic stem and progenitor cells (HSPCs) rapidly sense immune activation, yet their potential interplay with autoreactive T cells in MS is unknown. Qiang Liu, Department of Neurology, Institute of Neuroimmunology, Tianjin Medical University General Hospital, China, and his team reported that bone marrow HSPCs are skewed toward myeloid lineage concomitant with the clonal expansion of T cells in MS patients[3]. Lineage tracing in experimental autoimmune encephalomyelitis, a mouse model of MS, reveals remarkable bone marrow myelopoiesis with an augmented output of neutrophils and Ly6Chigh monocytes that invade the central nervous system (CNS). They found that myelin-reactive T cells preferentially migrate into the bone marrow compartment in a CXCR4-dependent manner. This aberrant bone marrow myelopoiesis involves the CCL5-CCR5 axis and augments CNS inflammation and demyelination (Fig.3). The study suggests that targeting the bone marrow niche presents an avenue to treat MS and other autoimmune disorders.
References
[1] iMSMS Consortium. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course [J]. Cell. 2022;185(19):3467-3486.e16. (IF=66.850)
[2] Lanz TV, Brewer RC, Ho PP, et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM [J]. Nature. 2022;603(7900):321-327. (IF=69.504)
[3] Shi K, Li H, Chang T, et al. Bone marrow hematopoiesis drives multiple sclerosis progression [J]. Cell. 2022;185(13):2234-2247.e17. (IF=66.850)
Cloud-Clone can not only provide a variety of animal models of autoimmune diseases, including allergic encephalomyelitis, Sjogren's syndrome, systemic lupus erythematosus, rheumatoid arthritis, etc., covering common autoimmune diseases. We also have various autoimmune disease detection indicators and the above CXCR4, CCL5, CCR5 and other related products, which can help researchers to carry out autoimmune disease research.