New findings in phage therapy research
Bacteriophages (phages) are viruses that selectively infect bacteria. Phages can be biologically active within bodies, infecting and subsequently killing and lysing bodyassociated bacteria, and the application of phages to bodies to combat bacterial infections is called phage therapy. However, the discovery of antibiotics, subsequent large-scale industrial production, and wide clinical application have caused phage therapy to become unfavorable. In recent years, phage therapy has been re-emphasized as the severity of drug-resistant bacterial infections has increased. Indeed, phages alone or combined with antibiotics have been successfully used to treat diferent bacterial infections, including bloodstream infection, lung infection, chronic otitis, skin burn infection, and enteric infection.
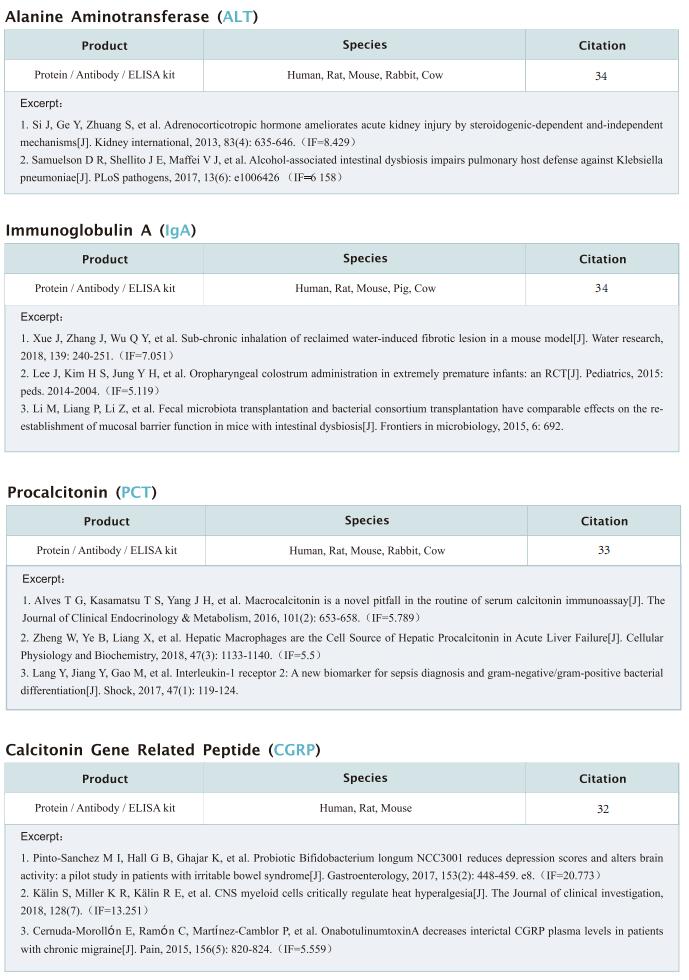
1. Architecture and self-assembly of the jumbo bacteriophage nuclear shell
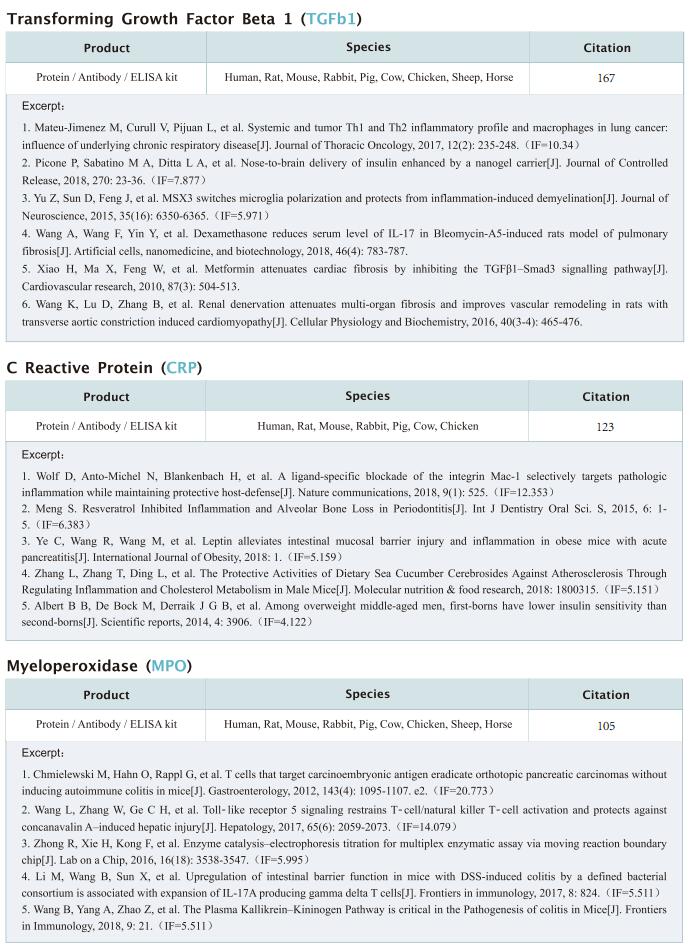
Bacteria encode myriad defences that target the genomes of infecting bacteriophage, including restriction-modification and CRISPR-Cas systems. In response, one family of large bacteriophages uses a nucleus-like compartment to protect its replicating genomes by excluding host defence factors. However, the principal composition and structure of this compartment remain unknown. Elizabeth Villa, Department of Molecular Biology, School of Biological Sciences, University of California San Diego, USA, and her team found that the bacteriophage nuclear shell assembles primarily from one protein, which they named chimallin[1]. Combining cryo-electron tomography of nuclear shells in bacteriophage-infected cells and cryo-electron microscopy of a minimal chimallin compartment in vitro, they showed that chimallin self-assembles as a flexible sheet into closed micrometre-scale compartments (Fig.1). The architecture and assembly dynamics of the chimallin shell suggest mechanisms for its nucleation and growth, and its role as a scaffold for phage-encoded factors mediating macromolecular transport, cytoskeletal interactions, and viral maturation. This work may provide help for the development of more effective bacteriophage therapy.
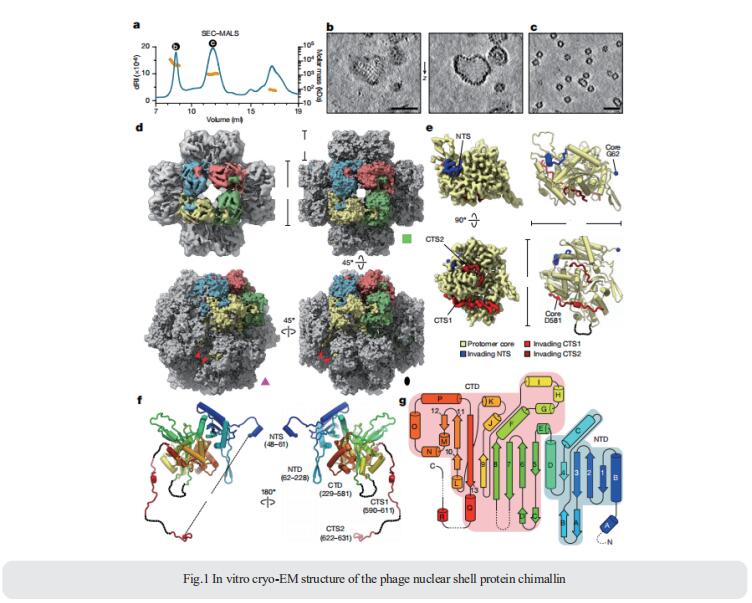
2. Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection
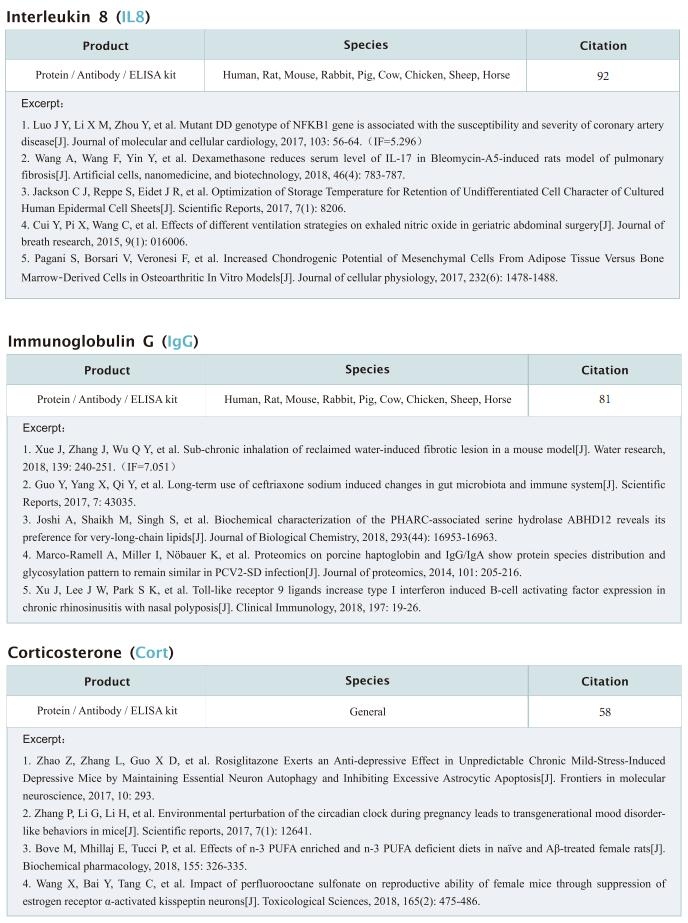
Persons with cystic fibrosis(pwCF) represent themost vulnerable population for nontuberculous mycobacteria (NTM) lung disease. M. abscessus infections have been associated with a greater decline in lung function compared with typical cystic fibrosis (CF) airway pathogens and when refractory to treatment imposes a contraindication to lung transplant. Jerry A. Nick, Department of Medicine, National Jewish Health, USA, and his team administered two phages intravenously to a male with treatment-refractory Mycobacterium abscessus pulmonary infection and severe cystic fibrosis lung disease (Fig.2)[2]. The phages were engineered to enhance their capacity to lyse M. abscessus and were selected specifically as the most effective against the subject’s bacterial isolate. In the setting of compassionate use, the evidence of phage-induced lysis was observed using molecular and metabolic assays combined with clinical assessments. M. abscessus isolates pre and post-phage treatment demonstrated genetic stability, with a general decline in diversity and no increased resistance to phage or antibiotics. The anti-phage neutralizing antibody titers to one phage increased with time but did not prevent clinical improvement throughout the course of treatment. The subject received lung transplantation on day 379, and systematic culturing of the explanted lung did not detect M. abscessus. The use of phages in very advanced lung disease with the objective to sufficiently control M. abscessus as a bridge to lung transplant may be considered based on this report.
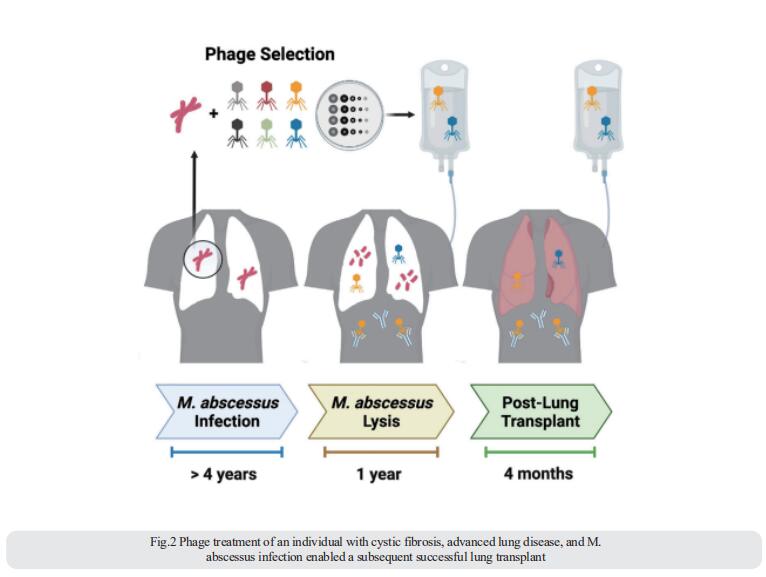
3. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation
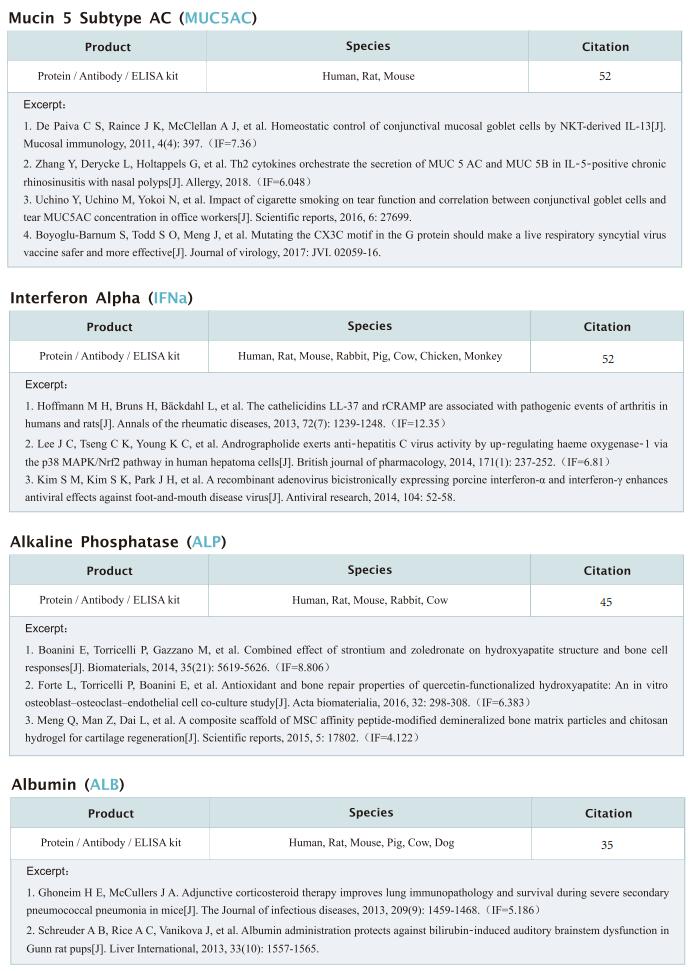
Human gut commensals are increasingly suggested to impact non-communicable diseases, such as inflammatory bowel diseases (IBD), yet their targeted suppression remains a daunting unmet challenge. Eran Elinav, Systems Immunology Department, Weizmann Institute of Science, Israel, and his team identified a clade of Klebsiella pneumoniae (Kp) strains, featuring a unique antibiotics resistance and mobilome signature, to be strongly associated with disease exacerbation and severity[3]. Transfer of clinical IBD-associated Kp strains into colitis-prone, germ-free, and colonized mice enhances intestinal inflammation. Stepwise generation of a lytic five-phage combination, targeting sensitive and resistant IBD-associated Kp clade members through distinct mechanisms, enables effective Kp suppression in colitis-prone mice, driving an attenuated inflammation and disease severity (Fig.3). Proof-of-concept assessment of Kp-targeting phages in an artificial human gut and in healthy volunteers demonstrates gastric acid-dependent phage resilience, safety, and viability in the lower gut. Collectively, they demonstrated the feasibility of orally administered combination phage therapy in avoiding resistance, while effectively inhibiting non-communicable disease-contributing pathobionts.
References
[1] Laughlin TG, Deep A, Prichard AM, et al. Architecture and self-assembly of the jumbo bacteriophage nuclear shell [J]. Nature. 2022, 608(7922):429-435. (IF=69.504)
[2] Nick JA, Dedrick RM, Gray AL, et al. Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection [J]. Cell. 2022, 185(11):1860-1874.e12. (IF=66.850)
[3] Federici S, Kredo-Russo S, Valdés-Mas R, et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation [J]. Cell. 2022, 185(16):2879-2898.e24. (IF=66.850)
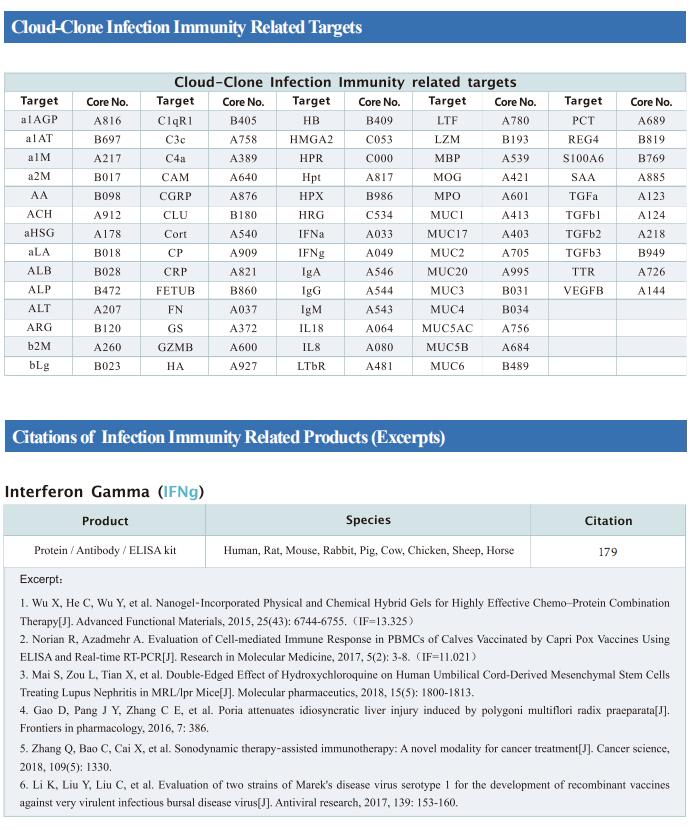
Cloud-Clone can provide a variety of commonly used infection immune detection indicators related products, including PCT, CRP, CGRP, IL-6, IL-8, IL-10, HMGB1, SAA, IFNα, etc., which can help the majority of scientific researchers to conduct infectious disease research.