New findings in ovarian cancer
Ovarian cancer (OC) is one of the most common gynaecological malignancies. Approximately 90% of primary malignant ovarian tumors are epithelial (carcinomas). The classification of ovarian epithelial tumors currently used by pathologists is based entirely on tumor cell morphology. The four major types of epithelial tumors: serous, endometrioid, clear cell, and mucinous. Often known as the silent killer, OC is frequently not diagnosed until it is at an advanced stage because of its generally vague symptoms, making it hard to treat on a curative basis. Whilst the survival of patients with advanced OC has increased over the last two decades through better surgery and morechemotherapy options. Most women with OC will suffer a tumour recurrence after first-line therapy and in almost all of them, resistance to chemotherapy will eventually develop leading to death from OC. Recently, multiple studies have reported on OC, which may provide help for the diagnosis and treatment of OC.
1. EZH2 mediated metabolic rewiring promotes tumor growth independently of histone methyltransferase activity in OC
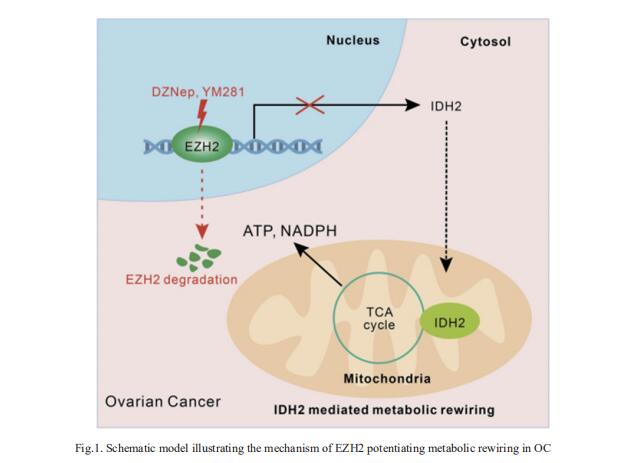
Enhancer of zeste homolog 2 (EZH2), the key catalytic subunit of polycomb repressive complex 2 (PRC2), is overexpressed and plays an oncogenic role in various cancers through catalysis-dependent or catalysis-independent pathways. However, the related mechanisms contributing to OC are not well understood. Jing Tan, State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center, China, and his team showed that a subgroup of OC patients with high EZH2 expression but low H3K27me3 exhibited the worst prognosis, with limited therapeutic options[1]. They demonstrated that induction of EZH2 degradation but not catalytic inhibition profoundly blocked OC cell proliferation and tumorigenicity in vitro and in vivo. Integrative analysis of genome-wide chromatin and transcriptome profiles revealed extensive EZH2 occupancy not only at genomic loci marked by H3K27me3 but also at promoters independent of PRC2, indicating a noncanonical role of EZH2 in OC. Mechanistically, EZH2 transcriptionally upregulated IDH2 to potentiate metabolic rewiring by enhancing tricarboxylic acid cycle (TCA cycle) activity (Fig.1), which contributed to the growth of OC. These data reveal a novel oncogenic role of EZH2 in OC and identify potential therapeutic strategies for OC by targeting the noncatalytic activity of EZH2.
2. Targeting the mevalonate pathway suppresses ARID1A-inactivated cancers by promoting pyroptosis
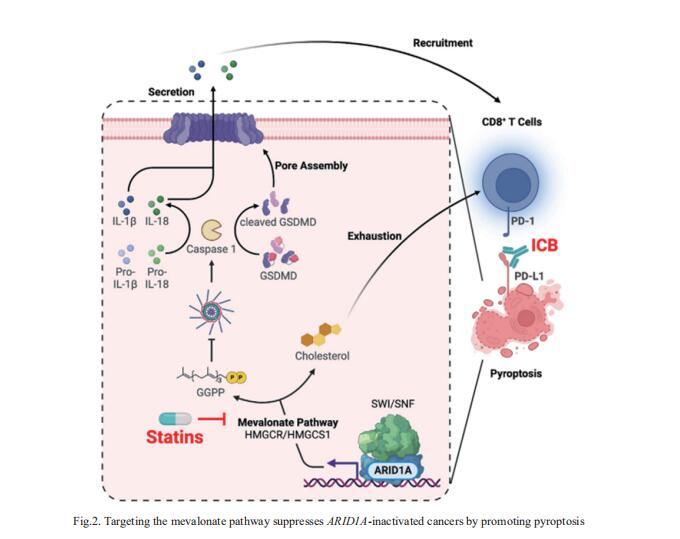
ARID1A, encoding a subunit of the SWI/SNF complex, is mutated in ~ 50% of clear cell ovarian carcinoma (OCCC) cases. Rugang Zhang, Immunology, Microenvironment and Metastasis Program, The Wistar Institute, USA, and his team showed that inhibition of the mevalonate pathway synergizes with immune checkpoint blockade (ICB) by driving inflammasome-regulated immunomodulating pyroptosis in ARID1A-inactivated OCCCs (Fig.2)[2]. SWI/SNF inactivation downregulates the rate-limiting enzymes in the mevalonate pathway such as HMGCR and HMGCS1, which creates a dependence on the residual activity of the pathway in ARID1A-inactivated cells. Inhibitors of the mevalonate pathway such as simvastatin suppresses the growth of ARID1A mutant, but not wild-type, OCCCs. In addition, simvastatin synergizes with anti-PD-L1 antibody in a genetic OCCC mouse model driven by conditional ARID1A inactivation and in a humanized immunocompetent ARID1A mutant patient-derived OCCC mouse model. Their data indicate that inhibition of the mevalonate pathway simultaneously suppresses tumor cell growth and boosts antitumor immunity by promoting pyroptosis, which synergizes with ICB in suppressing ARID1A-mutated cancers.
3. Tumor-secreted exosomal miR-141 activates tumor-stroma interactions and controls premetastatic niche formation in OC metastasis
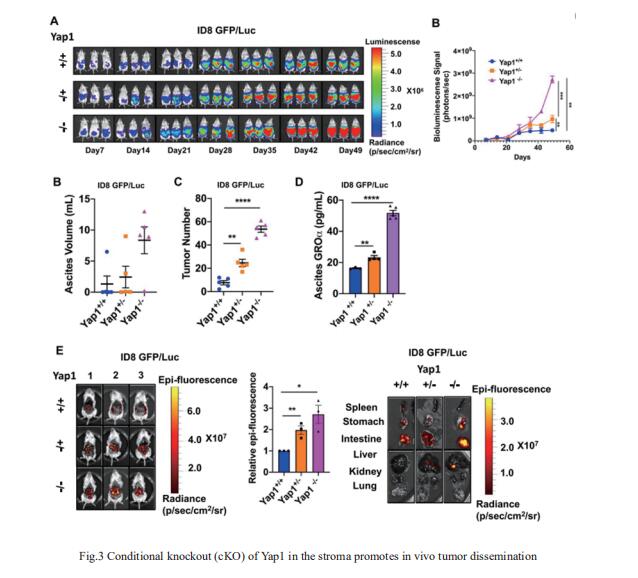
A pre-metastatic niche is required for metastatic colonization and is determined by tumor-stroma interactions, yet the mechanistic underpinnings remain incompletely understood. David W. Chan, Department of Obstetrics & Gynaecology, LKS Faculty of Medicine, The University of Hong Kong, China, and his team found that hsa-miR-141-3p (miR-141), an exosomal miRNA, is highly secreted by OC cells and reprograms stromal fbroblasts into proinfammatory cancer-associated fbroblasts (CAFs), facilitating metastatic colonization[3]. A mechanistic study showed that miR-141 targeted YAP1, a critical effector of the Hippo pathway, reducing the nuclear YAP1/TAZ ratio and enhancing GROα production from stromal fbroblasts. Stromal-specific knockout (cKO) of Yap1 in murine models shaped the GROα-enriched microenvironment, facilitating in vivo tumor colonization (Fig.3), but this effect was reversed after Cxcr1/2 depletion in OvCa cells. The YAP1/GROα correlation was demonstrated in clinical samples, highlighting the clinical relevance of this research and providing a potential therapeutic intervention for impeding premetastatic niche formation and metastatic progression of OCs.
References
[1]Chen J, Hong JH, Huang Y, et al. EZH2 mediated metabolic rewiring promotes tumor growth independently of histone methyltransferase activity in ovarian cancer. Mol Cancer. 2023;22(1):85. (IF=37.3)
[2]Zhou W, Liu H, Yuan Z, et al. Targeting the mevalonate pathway suppresses ARID1A-inactivated cancers by promoting pyroptosis. Cancer Cell. 2023;41(4):740-756.e10. (IF=50.3)
[3]Mo Y, Leung LL, Mak CSL, et al. Tumor-secreted exosomal miR-141 activates tumor-stroma interactions and controls premetastatic niche formation in ovarian cancer metastasis. Mol Cancer. 2023;22(1):4. (IF=37.3)
Cloud-Clone not only provides a variety of tumor experimental animal models, including tumor transplantation animal models, spontaneous tumor animal models, induced tumor animal models, tumor metastasis animal models, etc., covering common tumor research. We also have various cancer detection indicators and the above-mentioned EZH2, SWI/SNF, HMGCR, HMGCS1, YAP1, GROα and other related products, which can help scientific researchers to conduct cancer-related research.