New findings in myasthenia gravis research
Myasthenia gravis (MG) is an autoimmune disease causing neuromuscular junction (NMJ) impairment, characterized by weakness and easy fatigability on exertion involving different skeletal muscle districts. The autoimmune attack is mediated by autoantibodies targeting key functional and structural NMJ proteins: the acetylcholine receptor (AChR), the muscle-specific kinase receptor (MuSK) or the lowdensity lipoprotein receptor-related protein 4 (LRP4). Recommendations for MG treatment have been published. However, treatment improvement of myasthenic patients is still a medical need as a substantial proportion of them are refractory or intolerant. Recently, several papers have reported studies related to MG, which may contribute to the development of new treatment methods for MG.
1. MG-specific aberrant neuromuscular gene expression by medullary thymic epithelial cells in thymoma
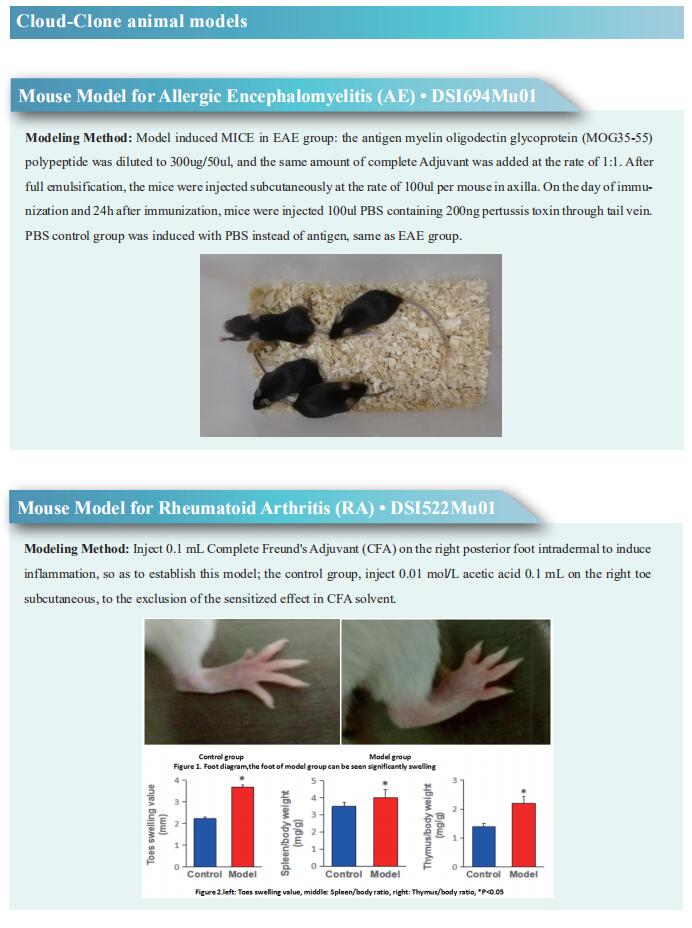
While MG frequently develops in thymoma patients, the etiologic factors for MG are not well understood. By constructing a comprehensive atlas of thymoma using bulk and single-cell RNA-sequencing, Tatsusada Okuno, Department of Neurology, Graduate School of Medicine, Osaka University, Japan, and his team identified ectopic expression of neuromuscular molecules in MG-type thymoma[1]. These molecules are found within a distinct subpopulation of medullary thymic epithelial cells (mTECs), which we name neuromuscular mTECs (nmTECs). MG-thymoma also exhibits microenvironments dedicated to autoantibody production, including ectopic germinal center formation, T follicular helper cell accumulation, and type 2 conventional dendritic cell migration (Fig.1). Cell-cell interaction analysis also predicts the interaction between nmTECs and T/B cells via CXCL12-CXCR4. The enrichment of nmTECs presenting neuromuscular molecules within MG-thymoma is further confirmed immunohistochemically and by cellular composition estimation from the MG-thymoma transcriptome. Altogether, this study suggests that nmTECs have a significant function in MG pathogenesis via ectopic expression of neuromuscular molecules.
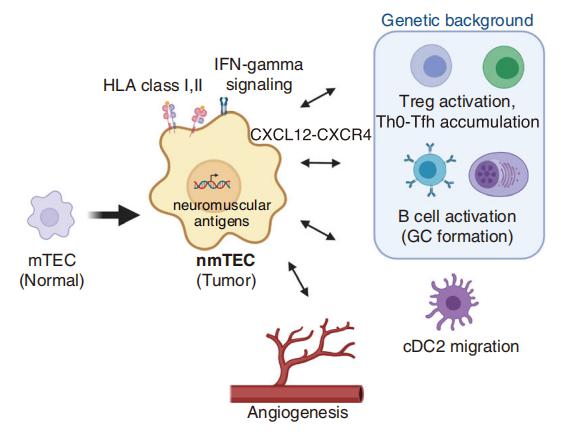
Fig.1 Proposed MG pathology in thymoma
2. Precision targeting of autoantigen-specific B cells in MuSK MG with chimeric autoantibody receptor T cells
MuSK MG is an autoimmune disease that causes life-threatening muscle weakness due to anti-MuSK autoantibodies that disrupt neuromuscular junction signaling. To avoid chronic immunosuppression from current therapies, Aimee S. Payne, Department of Dermatology, Perelman School of Medicine, University of Pennsylvania, USA, and his team engineered T cells to express a MuSK chimeric autoantibody receptor with CD137-CD3ζ signaling domains (MuSK-CAART) for precision targeting of B cells expressing anti-MuSK autoantibodies[2]. MuSK-CAART demonstrated similar efficacy as anti-CD19 chimeric antigen receptor T cells for depletion of anti-MuSK B cells and retained cytolytic activity in the presence of soluble anti-MuSK antibodies (Fig.2). In an experimental autoimmune MG mouse model, MuSK-CAART reduced anti-MuSK IgG without decreasing B cells or total IgG levels, refecting MuSK-specifc B cell depletion. These data contributed to an investigational new drug application and phase 1 clinical study design for MuSK-CAART for the treatment of MuSK autoantibody-positive MG.
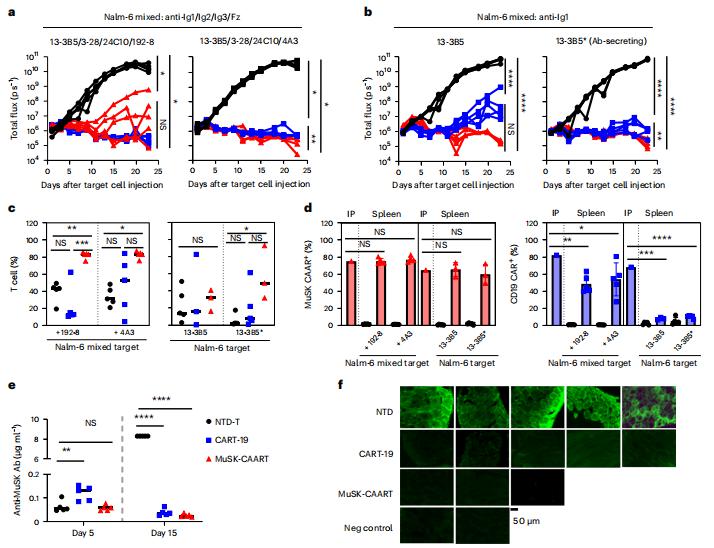
Fig.2 Targeting of anti-MuSK B cells through the BCR with MuSK-CAART demonstrates comparable efficacy as anti-CD19 CAR-mediated cytolysis
3. Receptor clustering and pathogenic complement activation in MG depend on synergy between antibodies with multiple subunit specifcities
Three mechanisms have been postulated by which autoantibodies might interfere with neurotransmission: direct antagonism of the receptor, complement-mediated destruction of the postsynaptic membrane, and enhanced internalization of the receptor. Using membrane antigen capture activated cell sorting (MACACS), Nicholas S. R. Sanderson, Department of Biomedicine, University Hospital Basel and University of Basel, Switzerland, and his team isolated AChR-specific B cells from patients with MG, and produced six recombinant antibodies[3]. All AChR-specific antibodies were hypermutated, including isotypes IgG1, IgG3, and IgG4, and recognized different subunits of the AChR. Despite clear binding, none of the individual antibodies showed significant antagonism of the AChR measured in an in vitro neuromuscular synapse model, or AChR-dependent complement activation, and they did not induce myasthenic signs in vivo. However, combinations of antibodies induced strong complement activation in vitro, and severe weakness in a passive transfer MG rat model, associated with NMJ destruction and complement activation in muscle (Fig.3). The strongest complement activation was mediated by combinations of antibodies targeting disparate subunits of the AChR, and such combinations also induced the formation of large clusters of AChR on the surface of live cells in vitro. They proposed that synergy between antibodies of different epitope specificities is a fundamental feature of this disease, and possibly a general feature of complement-mediated autoimmune diseases. The importance of synergistic interaction between antibodies targeting different subunits of the receptor can explain the well-known discrepancy between serum anti-AChR titers and clinical severity, and has implications for therapeutic strategies currently under investigation.
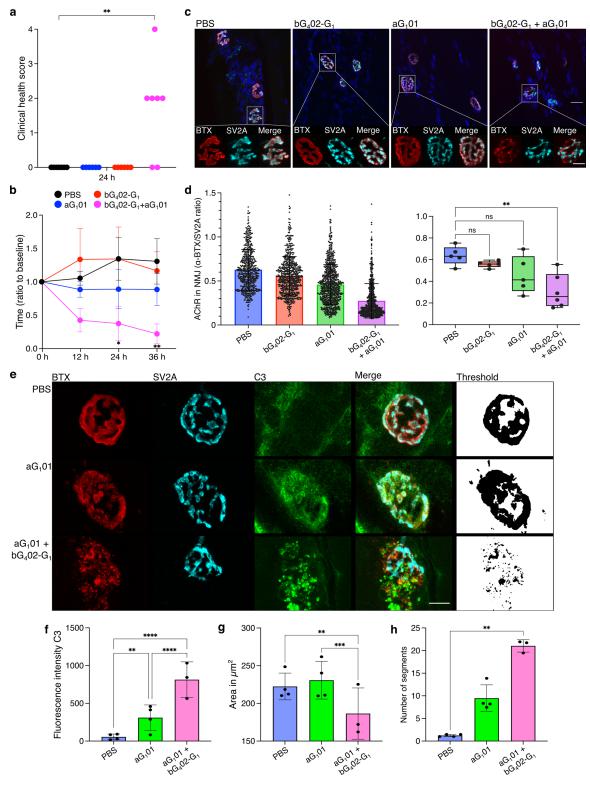
Fig.3 Influence of antibody combination on pathogenicity in a passive transfer MG rat model
References
[1]Yasumizu Y, Ohkura N, Murata H, et al. Myasthenia gravis-specific aberrant neuromuscular gene expression by medullary thymic epithelial cells in thymoma [J]. Nat Commun. 2022;13(1):4230. (IF=17.694)
[2]Oh S, Mao X, Manfredo-Vieira S, et al. Precision targeting of autoantigen-specific B cells in muscle-specific tyrosine kinase myasthenia gravis with chimeric autoantibody receptor T cells [J]. Nat Biotechnol. 2023;10.1038/s41587-022-01637-z. (IF=68.164)
[3]Rose N, Holdermann S, Callegari I, et al. Receptor clustering and pathogenic complement activation in myasthenia gravis depend on synergy between antibodies with multiple subunit specificities [J]. Acta Neuropathol. 2022;144(5):1005-1025. (IF=15.887)
Cloud-Clone can not only provide animal models of various autoimmune diseases, including myasthenia gravis, allergic encephalomyelitis, Sjogren's syndrome, systemic lupus erythematosus, rheumatoid arthritis, etc., covering common autoimmune diseases. We also have various autoimmune disease detection indicators and related products such as AChR, MuSK, LRP4, CXCL12, CXCR4, and CD19, which can help the majority of scientific researchers to carry out autoimmune disease research.