New Progress in the research of Anxiety Disorders
Anxiety disorders are among the most prevalent and disabling psychiatric disorders. The core feature of anxiety disorders is excessive fear and anxiety and related behavioral disturbances. Patients with anxiety disorders experience substantial physical and emotional discomfort and have elevated rates of substance use and medical illnesses. Furthermore, anxiety disorders are characterized by high comorbidity along with other mental disorders (eg, mood, substance-abuse, or personality disorders). Compounding this, response rates to first-line pharmacological and psychological treatments are less than 50%. Recently, several papers have reported anxiety related studies, which may provide help to determine the pathological mechanism and therapeutic targets of anxiety disorders.
1. A specific circuit in the midbrain detects stress and induces restorative sleep
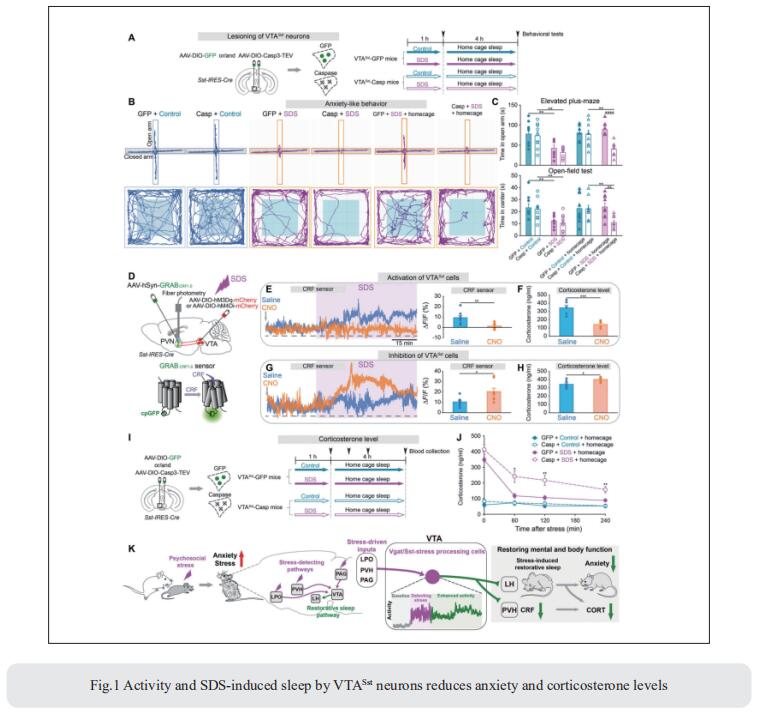
In mice, social defeat stress (SDS), an ethological model for psychosocial stress, induces sleep. Such sleep could enable resilience, but how stress promotes sleep is unclear. William Wisden, Department of Life Sciences, Imperial College London, UK, and his team found that a subset of ventral tegmental area GABA-somatostatin (VTAVgat-Sst) cells that sense stress and drive NREM and REM sleep via the lateral hypothalamus, and also inhibit corticotropin-releasing factor (CRF) release in the paraventricular hypothalamus[1]. Transient stress enhances the activity of VTAVgat-Sst cells for several hours, allowing them to exert their sleep effects persistently. Lesioning of VTAVgat-Sst cells abolished SDS-induced sleep; without it, anxiety and corticosterone levels remained elevated after stress (Fig.1). Thus, a specific circuit allows animals to restore mental and body functions via sleeping, potentially providing a refined route for treating anxiety disorders.
2. Cardiogenic control of affective behavioural state
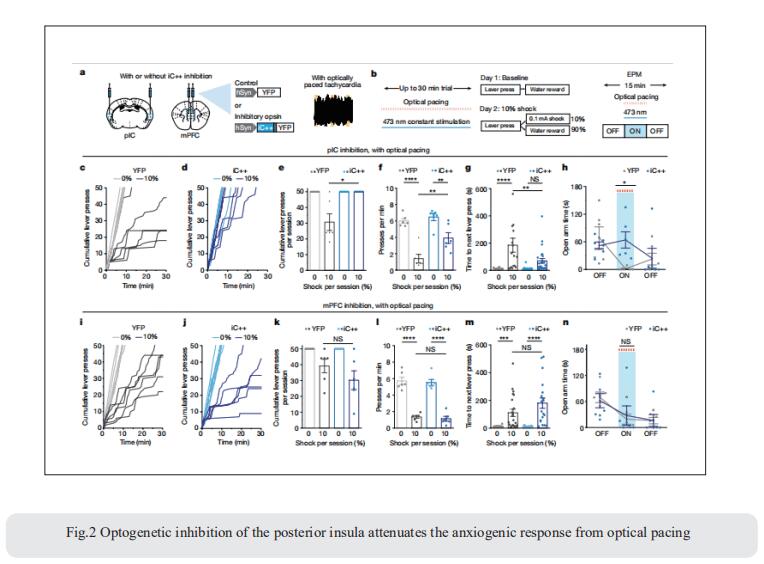
Emotional states influence bodily physiology, as exemplified in the top-down process by which anxiety causes faster beating of the heart. However, whether an increased heart rate might itself induce anxiety or fear responses is unclear. Karl Deisseroth, Department of Bioengineering, Stanford University, USA, and his team developed a noninvasive optogenetic pacemaker for precise, cell-type-specific control of cardiac rhythms of up to 900beats per minute in freely moving mice, enabled by a wearable micro-LED harness and the systemic viral delivery of a potent pump-like channelrhodopsin[2]. They found that optically evoked tachycardia potently enhanced anxiety-like behaviour, but crucially only in risky contexts, indicating that both central (brain) and peripheral (body) processes may be involved in the development of emotional states. To identify potential mechanisms, they used whole-brain activity screening and electrophysiology to find brain regions that were activated by imposed cardiac rhythms. They identified the posterior insular cortex as a potential mediator of bottom-up cardiac interoceptive processing, and found that optogenetic inhibition of this brain region attenuated the anxiety-like behaviour that was induced by optical cardiac pacing (Fig.2). Together, these findings reveal that cells of both the body and the brain must be considered together to understand the origins of emotional or affective states.
3. Distinct serotonergic pathways to the amygdala underlie separate behavioral features of anxiety
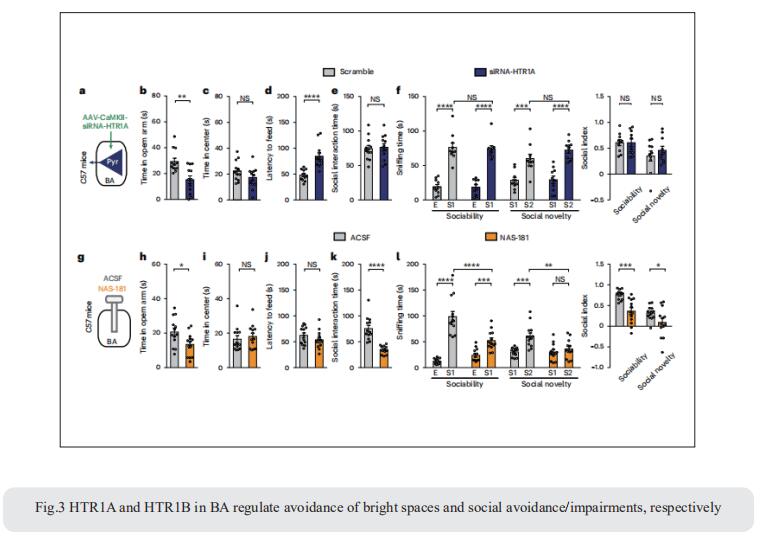
Anxiety-like behaviors in mice include social avoidance and avoidance of bright spaces. Whether these features are distinctly regulated is unclear. Xiao-Ming Li, Department of Neurobiology and Department of Neurology of Second Affiliated Hospital, Zhejiang University School of Medicine, China, and his team demonstrated that in mice, social and anxiogenic stimuli, respectively, increase and decrease serotonin (5-HT) levels in basal amygdala (BA)[3]. In dorsal raphe nucleus (DRN), 5-HT∩vGluT3 neurons projecting to BA parvalbumin (DRN5-HT∩vGluT3-BAPV) and pyramidal (DRN5-HT∩vGluT3-BAPyr) neurons have distinct intrinsic properties and gene expression and respond to anxiogenic and social stimuli, respectively. Activation of DRN5-HT∩vGluT3→BAPV inhibits 5-HT release via GABAB receptors on serotonergic terminals in BA, inducing social avoidance and avoidance of bright spaces. Activation of DRN5-HT∩vGluT3→BA neurons inhibits two subsets of BAPyr neurons via 5-HT1A receptors (HTR1A) and 5-HT1B receptors (HTR1B). Pharmacological inhibition of HTR1A and HTR1B in BA induces avoidance of bright spaces and social avoidance (Fig.3), respectively. These findings highlight the functional significance of heterogenic inputs from DRN to BA subpopulations in the regulation of separate anxiety-related behaviors.
References
[1]Yu X, Zhao G, Wang D, et al. A specific circuit in the midbrain detects stress and induces restorative sleep [J]. Science. 2022;377(6601):63-72. (IF=63.714)
[2]Hsueh B, Chen R, Jo Y, et al. Cardiogenic control of affective behavioural state [J]. Nature. 2023;615(7951):292-299. (IF=69.504)
[3]Yu XD, Zhu Y, Sun QX, et al. Distinct serotonergic pathways to the amygdala underlie separate behavioral features of anxiety [J]. Nat Neurosci. 2022;25(12):1651-1663. (IF=28.771)
Cloud-Clone can not only provide a variety of animal models of mental diseases, including anxiety, social defeat stress, schizophrenia, chronic stress depression, etc., covering common mental diseases research. We can also provide mental disease-related index detection products and the above-mentioned GABA, HTR1A and HTR1B related products, which can help the majority of scientific researchers to carry out mental diseases related research.

