A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin
On October 14, 2022, Cui Hua Liu, CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, China, and his team published a paper titled “A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin” in Science. Their data reveal a role of the pathogen-derived phospholipid phosphatase in the regulation of GSDMD-dependent pyroptosis and cytokine releases, extending understanding of the elaborate regulatory mechanism of cellular inflammasome-pyroptosis signaling pathways during pathogen infection.

The kits [ELISA Kit for Interferon Gamma (IFNg), SEA049Mu] of Cloud-Clone brand was chosed in this article, we are so proud for supporting the reaserchers.


NTRODUCTION: Pyroptosis is a proinflammatory form of programmed cell death characterized by membrane pore formation that allows the release of intracellular inflammatory mediators, a process that is elicited by the inflammasome-mediated cleavage and activation of gasdermin D (GSDMD). Growing evidence supports a critical role for pyroptosis in the control of infections by mammalian hosts, but how pathogens evade this immune response remains largely unexplored.
RATIONALE: Mycobacterium tuberculosis (Mtb), an ancient pathogen that causes tuberculosis (TB), has developed numerous intracellular survival strategies to evade host immunity and to drive the occurrence and development of TB. One notable feature evolved by Mtb is a set of eukaryotic-like effectors, but their host targets and regulatory roles in pathogen–host interactions remain largely unclear. In this study, we sought to identify the key pathogenic regulators of inflammasome-pyroptosis pathways from Mtb eukaryotic-like effectors, information that could improve our understanding of TB pathogenesis and provide potential targets for novel anti-TB treatment.
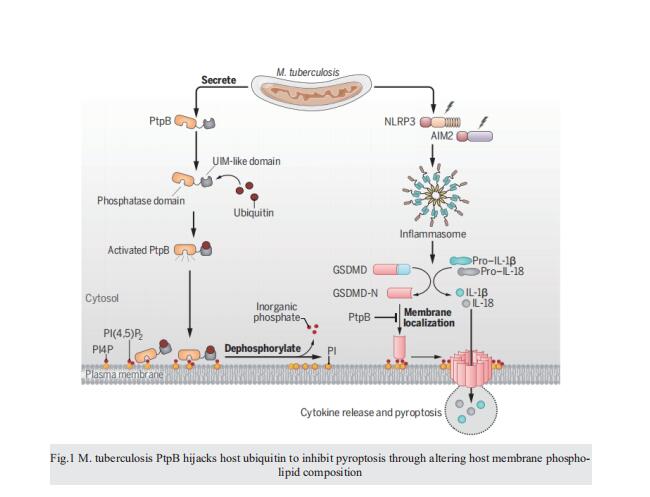
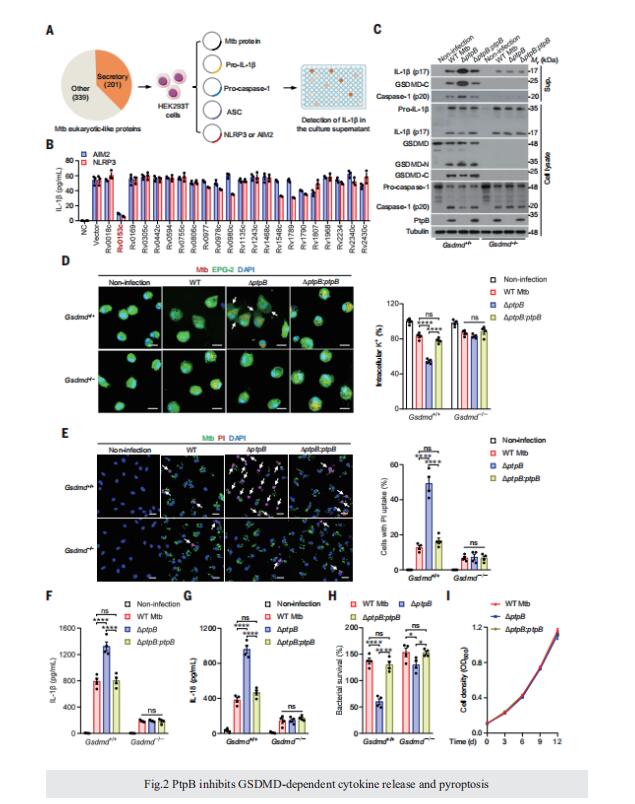
RESULTS: We examined the whole genome of Mtb to predict its secreted eukaryotic-like proteins possessing eukaryotic-like motifs or domains that might target host factors directly. These Mtb effector proteins were then subjected to further experimental analyses using an inflammasome reconstitution system for screening inhibitors of inflammasome-pyroptosis pathways. Out of 201 predicted Mtb-secreted eukaryotic proteins, six Mtb proteins (Rv0153c, Rv0561c, Rv0824c, Rv0861c, Rv1515c, and Rv1679) exhibited strong inhibitory effects on both NOD-like receptor protein 3 (NLRP3) and absent in melanoma 2 (AIM2) inflammasome pathways. Among these proteins, PtpB (i.e., Rv0153c) was most abundantly secreted by Mtb during infection. We thus focused on PtpB and further confirmed its inhibitory effect on AIM2 or NLRP3 inflammasome-mediated interleukin-1b (IL-1b) secretion. Subsequent experiments demonstrated that PtpB inhibited gasdermin D (GSDMD)–dependent cytokine release and pyroptosis to promote Mtb intracellular survival in macrophages. Mechanistically, Mtb-secreted PtpB could target and dephosphorylate host plasma membrane phosphatidylinositol-4-monophosphate (PI4P) and phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] to inhibit the membrane localization of the N-terminal cleavage fragment of GSDMD (GSDMD-N), thus preventing GSDMD-mediated immune responses. This phosphoinositide phosphatase activity requires binding of PtpB to ubiquitin. Accordingly, disrupting phospholipid phosphatase activity or the unusual ubiquitin-interacting motif (UIM)-like domain of PtpB markedly enhanced host innate immune responses and reduced intracellular pathogen survival in mice in a GSDMD-dependent manner.
CONCLUSION: We demonstrate that GSDMD-mediated pyroptosis and inflammatory cytokine release play a critical role in host anti-infection immunity, which is counteracted by Mtb effector protein PtpB. Our data reveal a role of the pathogen-derived phospholipid phosphatase in the regulation of GSDMD-dependent pyroptosis and cytokine releases, extending our understanding of the elaborate regulatory mechanism of cellular inflammasome-pyroptosis signaling pathways during pathogen infection. The present study also presents a strategy by which pathogens hijack ubiquitin to inhibit host pyroptosis by altering the phospholipid composition of the host membrane. Our discovery of the PtpB UIM-like domain, which is not homologous to any human protein, may provide potential selectivity for the development of anti-TB therapies.