Enzyme-linked Immunosorbent Assay Kit For Bovine Serum Albumin Residue
Instruction manual
First Edition (Revised on April, 2016)
[ INTENDED USE ]
The kit uses a competitive inhibition enzyme immunoassay technique for the in vitro quantitative measurement of Bovine Serum Albumin (BSA) residue in biological fluids.
[ SUMMARY AND EXPLANATION ]
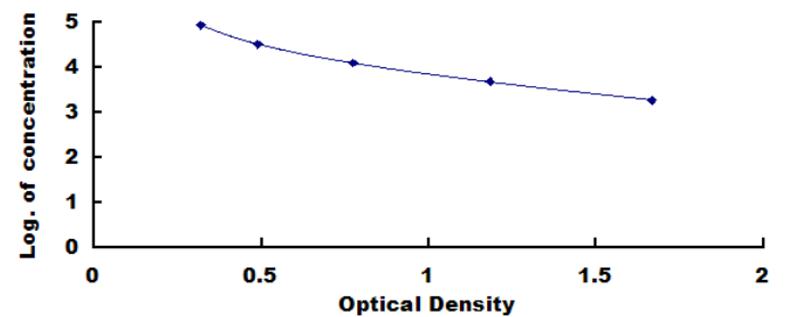
The manufacture of products by various biotechnological processes result in residual impurities of the desired product by components used in the culture media. When the products are intended to be used in human or animal, they should be highly purified to avoid potential health risks that might result from trace impurities. By using serum free media greatly reduces the impurities of biological products, but most commercial serum free media still contain certain amounts of albumin either of bovine, human or other species. This kit could be used to quantify BSA residues in cell and tissue culture media to 580.2ng/mL. The standard of BSA in the kit is calibrated by using standard from “National Drug Reference Standards”. The kit provides objective, precise and simple BSA quantification.
[ REAGENTS AND MATERIALS PROVIDED ]
Reagents | Quantity | Reagents | Quantity |
Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
Standard | 2 | Standard Diluent | 1×20mL |
Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
Detection Reagent B | 1×120μL | Assay Diluent B | 1×12mL |
TMB Substrate | 1×9mL | Stop Solution | 1×6mL |
Wash Buffer (30 × concentrate) | 1×20mL | Instruction Manual | 1 |
[ MATERIALS REQUIRED BUT NOT SUPPLIED ]
1. Microplate reader with 450 ± 10nm filter.
2. Single or multi-channel pipettes with high precision and disposable tips.
3. Microcentrifuge Tubes.
4. Deionized or distilled water.
5. Absorbent paper for blotting the microplate.
6. Container for Wash Solution.
7. 0.01mol/L (or 1×) Phosphate Buffered Saline(PBS), pH7.0-7.2.
[ STORAGE OF THE KITS ]
1. For unopened kit: All the reagents should be kept according to the labels on vials. The Standard, Detection Reagent A, Detection Reagent B and the 96-well strip plate should be stored at -20oC upon receipt while the others should be at 4oC.
2. For used kit: When the kit is used, the remaining reagents need to be stored according to the above storage condition. Besides, please return the unused wells to the foil pouch containing the desiccant pack, and zip-seal the foil pouch.
Note:
It is highly recommended to use the remaining reagents within 1 month provided this is prior to the expiration date of the kit. For the expiration date of the kit, please refer to the label on the kit box. All components are stable up to the expiration date.
[ SAMPLE COLLECTION AND STORAGE ]
Biological agents - Remove particulates by centrifugation and assay immediately or aliquot and store samples at ≤-20oC. Avoid repeated freeze-thaw cycles.
Note:
1. Samples to be used within 5 days may be stored at 4oC, otherwise samples must be stored at -20oC (≤1 month) or -80oC (≤2 months) to avoid loss of bioactivity and contamination.
2. When performing the assay, bring samples to room temperature.
[ REAGENT PREPARATION ]
1. Bring all kit components and samples to room temperature (18-25oC) before use. If the kit will not be used up in one time, please only take out strips and reagents for present experiment, and leave the remaining strips and reagents in required condition.
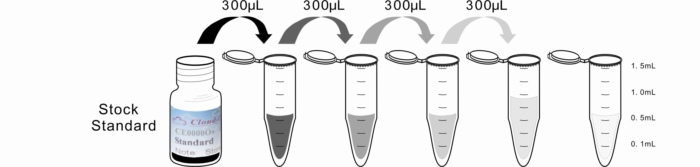
2. Standard - Reconstitute the Standard with 1.0mL of Standard Diluent, kept for 10 minutes at room temperature, shake gently(not to foam). The concentration of the standard in the stock solution is 100,000ng/mL. Please prepare 5 tubes containing 0.6mL Standard Diluent and produce a triple dilution series according to the picture shown below. Mix each tube thoroughly before the next transfer. Set up 5 points of diluted standard such as 100,000ng/mL, 33,333.3ng/mL, 11,111.1ng/mL, 3,703.7ng/mL, 1,234.6ng/mL, and the last EP tubes with Standard Diluent is the blank as 0ng/mL.
Matrix | Recovery range (%) | Average(%) |
serum(n=5) | 85-96 | 90 |
EDTA plasma(n=5) | 86-98 | 92 |
heparin plasma(n=5) | 96-105 | 101 |
[ LINEARITY ]
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of BSA and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
Sample | 1:2 | 1:4 | 1:8 | 1:16 |
serum(n=5) | 81-93% | 97-105% | 82-97% | 84-103% |
EDTA plasma(n=5) | 90-99% | 87-94% | 95-102% | 87-98% |
heparin plasma(n=5) | 83-97% | 82-92% | 80-104% | 85-101% |
Samples were diluted prior to assay as described in the SAMPLE PREPARATION section.
[ PRECISION ]
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level BSA were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level BSA were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
[ STABILITY ]
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
[ ASSAY PROCEDURE SUMMARY ]
1. Prepare all reagents, samples and standards;
2. Add 50μL standard or sample to each well.
And then add 50μL prepared Detection Reagent A immediately.
Shake and mix. Incubate 1 hour at 37oC;
3. Aspirate and wash 3 times;
4. Add 100μL prepared Detection Reagent B. Incubate 30 minutes at 37oC;
5. Aspirate and wash 5 times;
6. Add 90μL Substrate Solution. Incubate 10-20 minutes at 37oC;
7. Add 50μL Stop Solution. Read at 450 nm immediately.
[ IMPORTANT NOTE ]
1.Limited by the current conditions and scientific technology, we can't completely conduct the comprehensive identification and analysis on the raw material provided by suppliers. So there might be some qualitative and technical risks to use the kit.
2.The final experimental results will be closely related to validity of the products, so the kit should be used prior to the expiration date. And please store the kits exactly according to the instruction.
3. Kits from different batches may be a little different in detection range, sensitivity and color developing time. Please perform the experiment exactly according to the instruction attached in kit while electronic ones from our website is only for reference.
4. Do not mix or substitute reagents from one kit lot to another. Use only the reagents supplied by manufacturer.
5.Protect all reagents from strong light during storage and incubation. All the bottle caps of reagents should be covered tightly to prevent the evaporation and contamination of microorganism. TMB Substrate should remain colorless till it is reacted with the enzyme which binds to the microplate.
6.There may be some foggy substance in the wells when the plate is opened at the first time. It will not have any effect on the final assay results. Do not remove microplate from the storage bag until needed.
7. Wrong operations during the reagents preparation and loading, as well as incorrect parameter setting for the plate reader may lead to incorrect results. A microplate reader with a bandwidth of 10nm or less and an optical density range of 0-3 O.D. at 450 ± 10nm wavelength is acceptable for use in absorbance measurement. Please read the instruction carefully and adjust the instrument prior to the experiment.
8. Variation in sample preparation and each step of experimental operation may cause different results. In order to get better reproducible results, the operation of each step in the assay should be controlled.
9. Each kit has been strictly passed Q.C test. However, results from end users might be inconsistent with our in-house data due to some unexpected transportation conditions or different lab equipments. Intra-assay variance among kits from different batches might arise from above factors, too.
10. Kits from different manufacturers with the same item might produce different results, since we haven’t compared our products with other manufacturers.
11. The standard of the kit and immunogen used for antibody preparation are commonly recombinant proteins, as different fragments, expression systems, purification methods might be used in recombinant protein preparation, we can not guarantee the kit could detect recombinant protein from other companies. So, it is not recommended to use the kit for the detection of recombinant protein.
12. Please predict the concentration of target molecules in samples, or arrange a preliminary experiment, it is a good way to solve specific problem, e.g. the concentration of samples are beyond the detection range of the kit.
13. The kit might not be suitable for detection of samples from some special experiment, for instance, knock-out experiments, due to their uncertainty of effectiveness.
14. The instruction manual is also for the kit of 48T, but all reagents of 48T kit are reduced by half.
15. The kit is designed for research use only, we will not be responsible for any issue if the kit was used in clinical diagnostic or any other procedures.
[ PRECAUTION ]
The Stop Solution suggested for use with this kit is an acid solution. Wear eye, hand, face, and clothing protection when using this material.
[ TROUBLE SHOOTING ]
Problem | Possible Source | Correction Action |
Poor Standard Curve | Improper standard curve preparation | Ensure accurate operation of the dilution |
Incomplete washing and aspiration | Adequate washing and adequate aspiration | |
Inaccurate Pipetting | Check and Calibrate pipettes | |
Poor Precision | Incomplete washing of wells | Ensure sufficient washing |
Inadequate mixing and aspiration reagents | Adequate aspiration and mixing reagents | |
Reused pipette tips, containers and sealers | Change and use new pipette tips, containers and sealers | |
Inaccurate Pipetting | Check and Calibrate pipettes | |
Low O.D Values | Inadequate reagent volumes added to wells | Calibrate pipettes and Add adequate reagents |
Incorrect incubation times | Ensure sufficient incubation times | |
Incorrect incubation temperature | Reagents balanced to room temperature | |
Conjugate or substrate reagent failure | Mix conjugate & substrate, color should develop immediately | |
No stop solution added | Follow the assay protocol in the kit manual | |
Read beyond suggested reading time | Read within the time recommended in the manual | |
Sample Values | Improper Sample Storage | Store the sample properly and use the fresh sample |
Improper sample collection and preparation | Take proper sample collection and preparation method | |
Low quantity of analyte in samples | Use new sample and repeat assay |